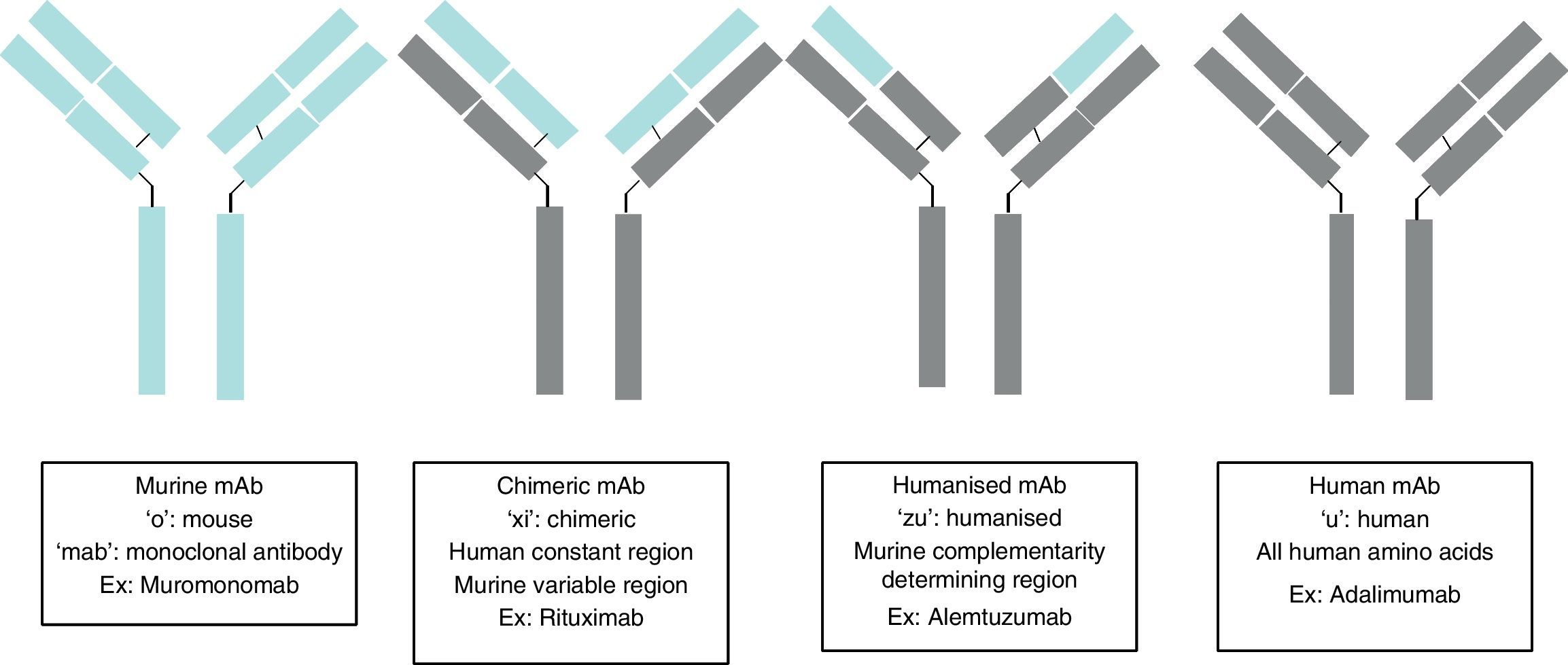
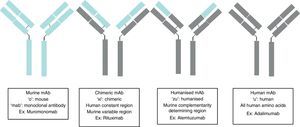
Monoclonal antibodies (mAb) are immunoglobulins specially designed to act against specific targets, in such a way that their administration stops a specific pathogenic process, stimulates a particular cellular action, or changes a cell mechanism to another pathway of interest. Their production is based on the establishment of modified immortal B lymphocytes to produce a specific immunoglobulin. Depending on the level of purity, this immunoglobulin may be murine complement (ending in “o”, for example muromonab); chimeric, in which all the immunoglobulin is human, except in the variable region which is murine (ending in “xi”, for example, rituximab); humanised, in which all the immunoglobulin is human, except in the variable complement region which remains murine (ending in “zu”, for example, natalizumab); and human complement (ending in “u”, for example, adalimumab). Therefore, there will be two types of secondary effects: those arising from the action of the antibody, such as opportunistic infections due to immunosuppression, and those arising from the administration of a protein, such as anaphylactic reactions. The sources used for the present articles were articles published in PubMed, located by searching for “Monoclonal antibodies and Secondary effects”, and the web pages of the European Medicines Agency (EMEA) and the US Food and Drugs Administration (FDA).
DevelopmentThe secondary effects arising from the mechanisms of action were opportunistic infections, common infections, development of tumours and autoimmune phenomena, and those arising from the administration of proteins: anaphylactic reaction, cytokine release syndrome, and the development of neutralising antibodies (NAb). Finally, the management of mAb in clinical practice and in special situations is discussed, including administering vaccines, pregnancy and paediatric use. Reference will be made to immune recovery syndrome.
ConclusionsmAb are highly effective drugs when specifically indicated, but they also may incur serious secondary effects, which although incidence is low, require close monitoring of the patients receiving these treatments.
Los anticuerpos monoclonales son inmunoglobulinas diseñadas específicamente para actuar frente a dianas concretas, de forma que su administración interrumpa un proceso patogénico concreto, estimule una acción celular determinada o desvíe un mecanismo celular hacia una vía de interés. Su producción se basa en el establecimiento de linfocitos B inmortales modificados para producir una inmunoglobulina específica; según el nivel de pureza de la inmunoglobulina, ésta puede ser: completamente murina (terminación en «o», ejemplo muromonomab); quimérica, donde toda la inmunoglobulina es humana, salvo la región variable que es murina (terminación en «xi», ejemplo el rituximab); humanizada, donde toda la inmunoglobulina es humana salvo la región complementaria variable que sigue siendo murina (terminación en «zu», ejemplo el natalizumab); y completamente humana (terminación en «u», ejemplo adalimumab). Los efectos secundarios serán pues de dos tipos: los derivados de la acción del anticuerpo como infecciones oportunista por inmunosupresión y los derivados de la administración de una proteína como es la aparición de una reacción anafiláctica. Las fuentes utilizadas para el presente artículo ha sido artículos publicados en PubMed, tras realizar una búsqueda «Monoclonal antibodies and Secondary effects», y las páginas web de la Agencia Europea del Medicamento (EMEA) y la Administración para la Alimentación y Fármacos (FDA).
DesarrolloSe describen los efectos secundarios derivados de los mecanismos de acción: infecciones oportunistas, infecciones comunes, desarrollo de tumores y desarrollo de fenómenos autoinmunes. Así como los efectos secundarios derivados de la administración de proteínas: reacción anafiláctica, síndrome de liberación de citocinas y desarrollo de anticuerpos neutralizantes. Finalmente, se desarrolla el manejo de los anticuerpos monoclonales en la práctica clínica y el manejo de situaciones especiales: administración de vacunas, embarazo y edad pediátrica. Con una referencia al síndrome de reconstitución inmune.
ConclusionesLos anticuerpos monoclonales son fármacos altamente eficaces en las condiciones de indicación específica pero con un perfil de efectos secundarios graves aunque poco incidentes, lo que requiere de una estrecha vigilancia de los pacientes que están con estos regímenes terapéuticos.
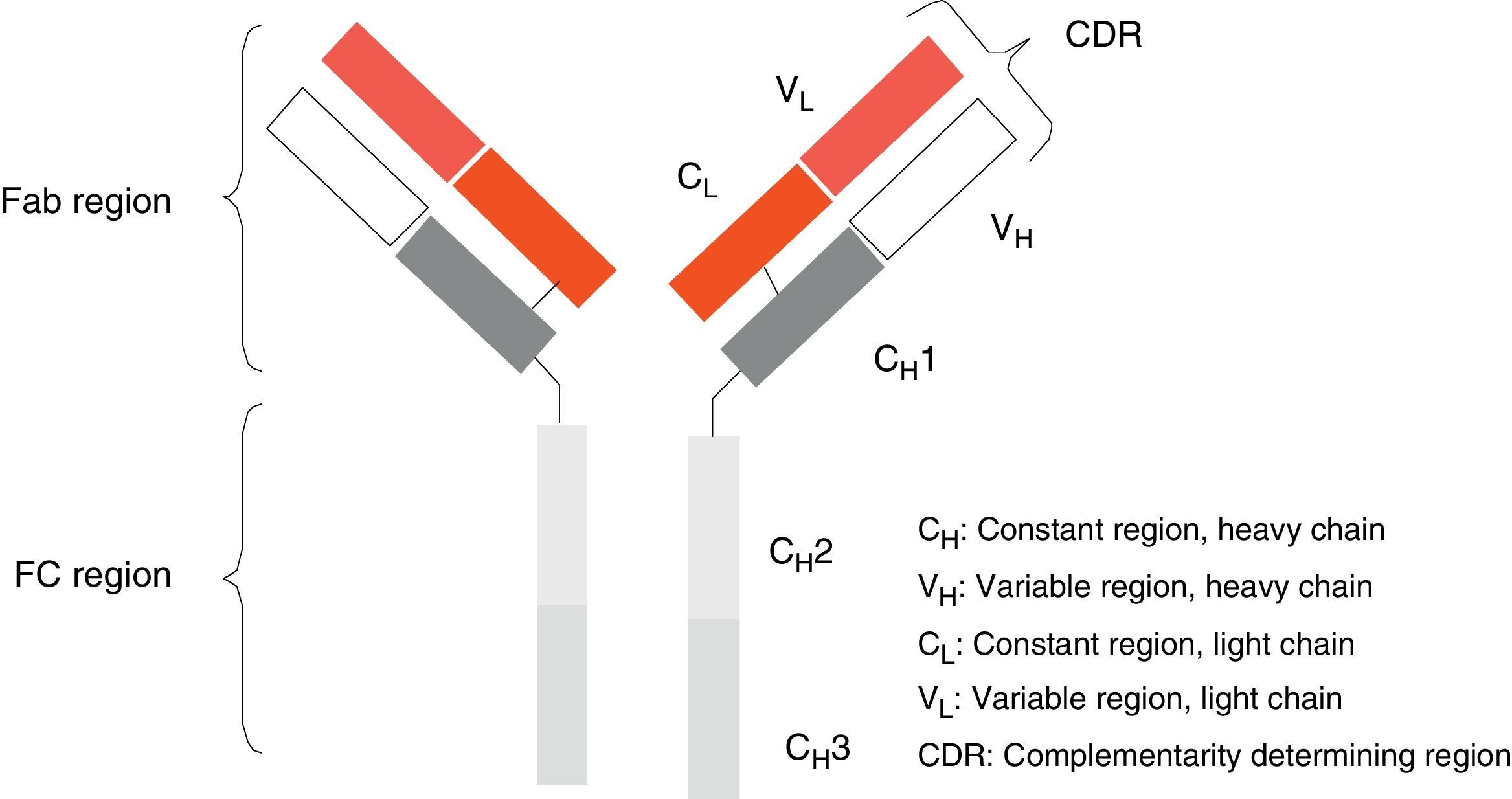
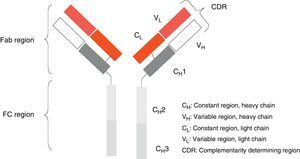
Monoclonal antibodies (mAb) are a type of ‘customised’ drug; the idea behind such drugs is that current technological abilities enable production of antibodies directed against specific antigens. Monoclonal antibodies are immunoglobulins (Fig. 1) which have been modified in order to enable them to exert a specific and controlled effect on cells and their functions (Fig. 2).1
Georges Köhler and César Milstein first began producing mAbs in 1975.2 They generated a stable cell line that secreted a specific immunoglobulin isotype targeting a certain antigen as a result of fusing 2 different cells using physical and chemical techniques (polyethylene glycol and centrifugation). The purpose of the process is to create a hybridoma,3,4 that is, an immunoglobulin-producing immortalised cell obtained by fusing a B cell previously immunised against the antigen being targeted and an immortal myeloma cell selected for its absence of antibody secretion and lack of the hypoxanthine-guanine phosphoribosyltransferase (HGPRT) enzyme.
Ever since the first mAb was employed to treat primary transplant rejection, doctors observed that these drugs caused a severe hypersensitivity reaction.5 Techniques were therefore developed to minimise that effect. The first human mAbs were generated in 1985 using recombinant DNA technology. In this process, genes encoding the Ig variable region in mice were joined with genes encoding the constant region in humans. These genes were later inserted into myelomas, where they produced new Ab molecules containing a human component and a mouse component (Fab fraction). These molecules, known as chimeric mAbs, are less immunogenic than mouse mAbs but also have a lower capacity for Ab production. Scientists began using the technique of humanising antibodies in 1986 so as to minimise the mouse antibody components that were provoking the immune response. Humanised mAbs are produced by protein engineering.6 During this process, the CDRs from mouse Ig are transferred to the human immunoglobulin heavy chain variable region. Although humanising mAbs has minimised the anti-mAb immune response, anaphylactic reactions are still reported in up to 9% of the cases.7
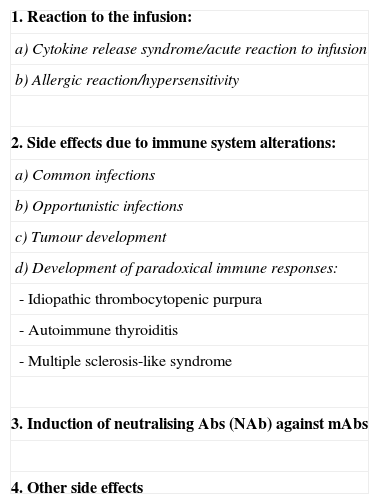
From the above, we can conclude that typical side effects of treatment with mAbs fall into 2 basic categories: those arising from the specific action of the antibodies (immunosuppression) and those arising from the presence of proteins that may provoke different degrees of hypersensitivity (Table 1).
General side effects of mAbs.
| 1. Reaction to the infusion: |
| a) Cytokine release syndrome/acute reaction to infusion |
| b) Allergic reaction/hypersensitivity |
| 2. Side effects due to immune system alterations: |
| a) Common infections |
| b) Opportunistic infections |
| c) Tumour development |
| d) Development of paradoxical immune responses: |
| - Idiopathic thrombocytopenic purpura |
| - Autoimmune thyroiditis |
| - Multiple sclerosis-like syndrome |
| 3. Induction of neutralising Abs (NAb) against mAbs |
| 4. Other side effects |
Monoclonal antibodies constitute a therapeutic group with a continuously expanding list of indications. The intrinsic characteristics of patients who are candidates for treatment, added to the effect and composition of these drugs, mean that we may face a wide range of side effects ranging from anaphylactic reaction to neoplasia. As a result, the following factors must be contemplated before administering treatment: prior immunosuppressive therapy; prior history of infections, especially viral infections from the herpes family (zoster or simple), and immunological state with respect to hepatitis B and C virus; tumour history; and any prior immunological changes such as autoimmune thyroiditis. In general, doctors recommend performing a Mantoux test, serology for hepatitis B and C, white blood cell counts (CD4 and CD8), and a complete blood count.
The side effects that may appear are classified as follows: reactions occurring shortly or immediately after treatment; infections, whether community-acquired or opportunistic; reactivation or development of autoimmune phenomena, including production of neutralising antibodies (NAb); and appearance of neoplasia.
Lastly, whether during or after mAb treatment, we generally face 2 problems: vaccination of treated patients, and possible reactivation of the process being treated.
Reactions to mAb infusionEither during or immediately after the administration of a mAb, a patient may display either of 2 types of infusion reaction (IR): massive cytokine release syndrome, or hypersensitivity reaction.
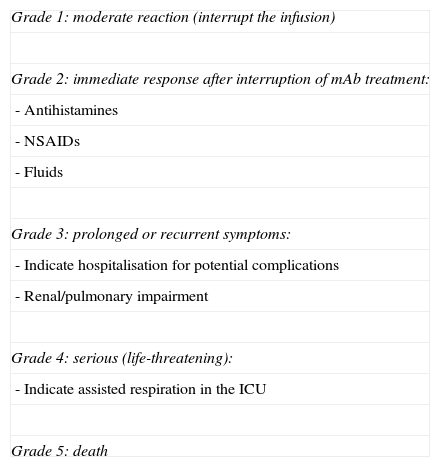
Cytokine release syndrome (CRS): acute reaction to infusionCRS is a systemic syndrome characterised by the appearance of arthralgia with myalgia, fever, headache, respiratory changes, hiccups or hypertension, nausea, vomiting, skin rash with scaling and pruritus, sweating, and tachycardia. This dynamic syndrome develops in 3 phases (Table 2). Management of CRS depends on the degree of toxicity that is reached (Table 3).8–11
Phases in the development of CRS/ARI.
| Phase 1: (60–90minutes after infusion): |
| - Lumbar pain |
| - Gastrointestinal symptoms |
| - Rigidity, erythema |
| - Peripheral vasodilation |
| Phase 2: (4 hours): |
| - Hypotension, tachycardia |
| - Fever |
| - Lymphocytopenia, monocytopenia |
| Phase 3: (16–20 hours): |
| - Multiple organ dysfunction and DIC |
Criteria for CRS/ARI toxicity and management.
| Grade 1: moderate reaction (interrupt the infusion) |
| Grade 2: immediate response after interruption of mAb treatment: |
| - Antihistamines |
| - NSAIDs |
| - Fluids |
| Grade 3: prolonged or recurrent symptoms: |
| - Indicate hospitalisation for potential complications |
| - Renal/pulmonary impairment |
| Grade 4: serious (life-threatening): |
| - Indicate assisted respiration in the ICU |
| Grade 5: death |
The frequency with which CRS presents depends on the type of mAb and ranges from less than 0.2% in cases treated with bevacizumab to 10% with infusions of rituximab. The origin of this syndrome is found in the mobilisation of effector cells to the location where mAb acts on its target, a process which releases cytokines IL-6 and TNF-α.12
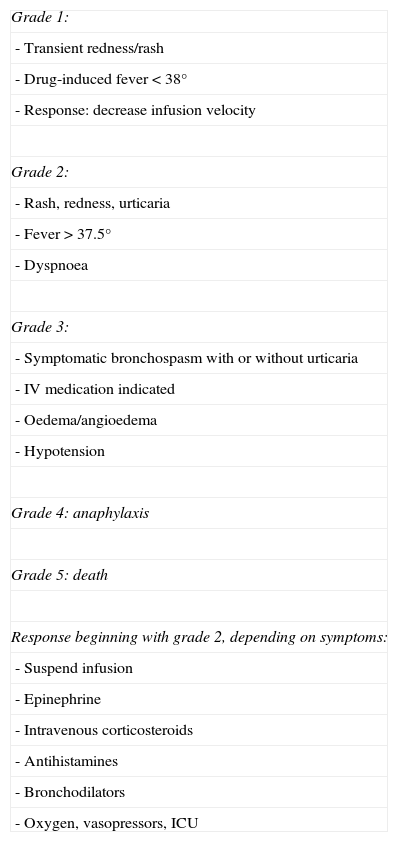
Hypersensitivity reactionHR is a systemic reaction characterised by dyspnoea, bronchospasm, fever, hiccups or arterial hypertension, oedema/angioedema, and dermatological symptoms ranging from mild rash to severe urticaria. HR is a type-1 reaction (mediated by IgE); for this reaction to occur, the subject had to have been exposed to the antigen previously. It is therefore more frequent during the second or third infusion, although some cases describe a later onset. Its mechanism is a release of histamine, leukotrienes, and prostaglandins in addition to cytokines, principally TNF-α.13,14 As in CRS, varying degrees of toxicity have been described according to clinical symptoms, and different courses of action should be taken depending on toxicity (Table 4).15,16
Criteria for HR toxicity and management.
| Grade 1: |
| - Transient redness/rash |
| - Drug-induced fever<38° |
| - Response: decrease infusion velocity |
| Grade 2: |
| - Rash, redness, urticaria |
| - Fever>37.5° |
| - Dyspnoea |
| Grade 3: |
| - Symptomatic bronchospasm with or without urticaria |
| - IV medication indicated |
| - Oedema/angioedema |
| - Hypotension |
| Grade 4: anaphylaxis |
| Grade 5: death |
| Response beginning with grade 2, depending on symptoms: |
| - Suspend infusion |
| - Epinephrine |
| - Intravenous corticosteroids |
| - Antihistamines |
| - Bronchodilators |
| - Oxygen, vasopressors, ICU |
The mAb drug group has very specific therapeutic targets, but even so, the drugs do alter the immune system. This results in a state of immunosuppression that provokes increased susceptibility to common infections and the appearance of opportunistic infections. At the same time, the functional change in the immune system has consequences on the normal immune system response to malignant cells. At least in theory, this could mean a higher probability of tumour development. Lastly, changes in immunity result in paradoxical responses, and unexpected autoimmune manifestations may appear.
New and community-acquired infectionsThe general mechanism of response to microorganisms depends on activation of humoral immunity in the presence of encapsulated organisms (Streptococcus, Neisseria meningitidis, Haemophilus) or fungi, or on activation of the cellular immune response to bacteria, viruses, protozoans, and fungi.17,18
Risks associated with mAb use can be summarised as follows: increased incidence of community-acquired pneumonia; increase in viral infections of the upper respiratory tract, adenovirus, and respiratory syncytial virus; and increased incidence of infections related to encapsulated bacteria. Development of urinary sepsis, especially in patients treated with rituximab, has resulted in death in some cases.
An additional problem is the possibility of reactivation of latent tuberculosis, which has occurred with anti-TNF alpha therapy for rheumatoid arthritis19,20 or for hepatitis B virus.
Opportunistic infectionsOf the many different opportunistic infections that have been reported during mAb treatment, there are 2 which interest us from a neurological perspective: herpes virus family infections and JC virus infections.
Herpes virus family infectionsInfection with varicella zoster virus may occur during NTZ treatment (1%–2% of cases). The infection may appear as chickenpox, which requires specific treatment, but its presence does not require withdrawing mAbs. In other reported cases, it appears as herpes zoster, which may cause death; another report describes a fatal case of herpesviral encephalitis. The literature describes a case of encephalitis caused by HHV-6 and reactivations of that virus in patients treated with NTZ, as well as during treatment with alemtuzumab and rituximab.21–23
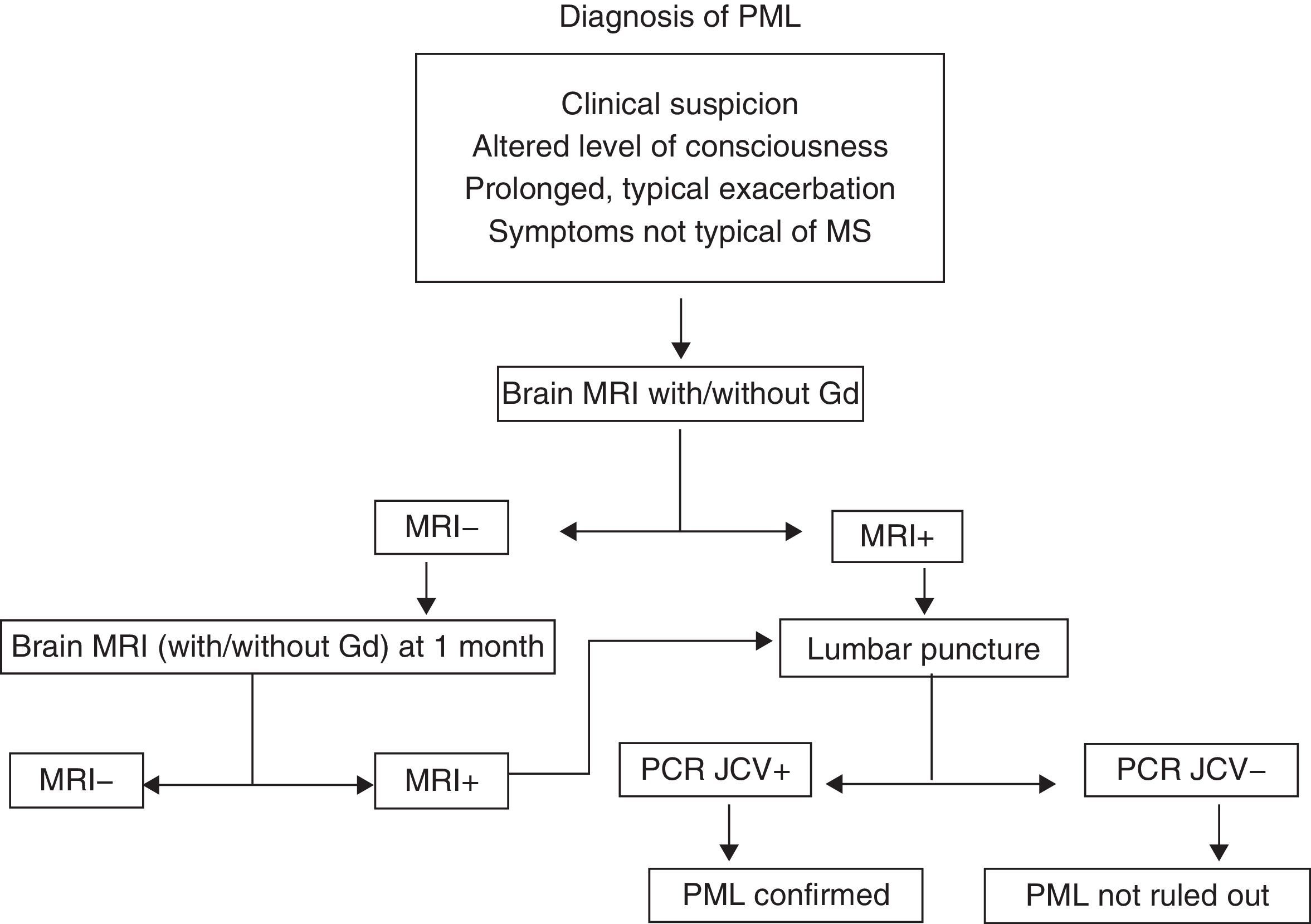
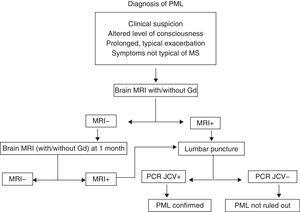
Progressive multifocal leucoencephalopathy (PML)PML is an infection with a high mortality rate (60%) that leads to disability in almost all patients. It is caused by the JC virus, which is ubiquitous. More than 60% of all people have antibodies to that virus, which is why there are no diagnostic tests that would allow us to define risk patterns based on prior contact.24–27 The increased frequency of infections with this specific virus in patients treated with mAb, especially NTZ, has not yet been explained. At present, we can only maintain a high level of suspicion and closely monitor patients undergoing treatment with NTZ or indeed with any other type of mAb. Cases have been described with rituximab,28 infliximab, and even non-specific immunosuppressants such as mycophenolate mofetil. For suspected cases of PML, doctors recommend an MRI and CSF studies. If the patient is being treated with NTZ, that drug should be suspended and the patient will require plasmapheresis and a specific antiviral treatment even before CSF results have confirmed the infection.29 At present, there is no consensus for treatment; options include mefloquine, cytarabine and cidofovir, and mirtazapine may be used as adjuvant treatment (Fig. 3). The frequency of PML in patients treated with natalizumab seems to increase with time of exposure to the drug. The incidence rate is currently calculated at 1.55 cases per 1000 patients, among patients who have completed more than 24 months of treatment (SD 1.06–2.19).
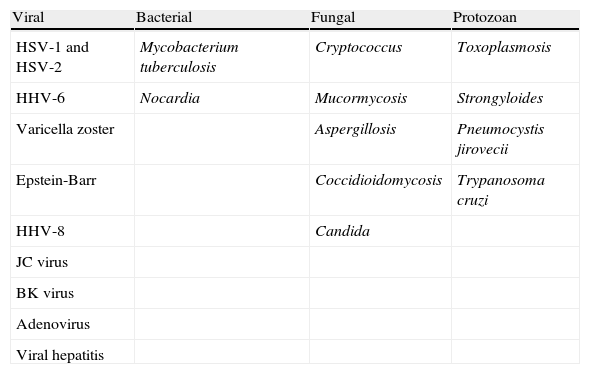
Other opportunistic infectionsWhile undergoing treatment with NTZ, patients with Crohn's disease who had previously been treated with an immunosuppressant (azathioprine) developed toxoplasmosis, cytomegalovirus, candidiasis, aspergillosis, pneumonia due to Pneumocystis jirovecii, and tuberculous peritonitis. The literature also describes (fatal) cases of colitis due to Clostridium difficile during rituximab treatment (Table 5).
Most common opportunistic infections seen with mAb use.
| Viral | Bacterial | Fungal | Protozoan |
| HSV-1 and HSV-2 | Mycobacterium tuberculosis | Cryptococcus | Toxoplasmosis |
| HHV-6 | Nocardia | Mucormycosis | Strongyloides |
| Varicella zoster | Aspergillosis | Pneumocystis jirovecii | |
| Epstein-Barr | Coccidioidomycosis | Trypanosoma cruzi | |
| HHV-8 | Candida | ||
| JC virus | |||
| BK virus | |||
| Adenovirus | |||
| Viral hepatitis |
HHV: human herpes virus; HSV: herpes simplex virus.
The possibility of a patient's developing neoplasia during treatment with mAbs is controversial. Some data indicate that there may be an increased risk of developing certain types of neoplasia. Initial reports pointed to a higher incidence of solid tumours among patients treated with infliximab, but a meta-analysis revealed no differences with respect to the general population or patients treated with methotrexate. Regarding neoplasia development among patients treated with natalizumab, the pivotal trial in monotherapy reported neoplasia incidence of 0.79% vs. 0.39% in the placebo group. During its commercialisation period, 2 cases of melanomas developing during treatment were detected.30 Based on the above, the drug is not recommended for patients with a family or personal history of melanomas, visible nevus or atypical ephelides. Solid organ neoplasias affecting the bladder, colon, and lungs have been observed in patients undergoing natalizumab treatment, whether for Crohn's disease or for multiple sclerosis.
Interleukin 12/23 (ustekinumab), which is still being developed, is effective against psoriasis, Crohn's disease, and multiple sclerosis, but it may have neoplastic potential. IL-12 promotes infiltration by cytotoxic T cells, added to the fact that IL-23 induces an inflammatory process promoted by tumours.31–33 No such cases have been reported in trials of alemtuzumab, daclizumab, or rituximab.
Development of neutralising antibodies (NAb)Monoclonal antibodies are proteins, and as such they behave like antigens. As a result, the body may trigger an immune response to those antigens by generating antibodies to the mAbs being delivered.
Two types of Ab have been described: the first type includes binding antibodies that, generally speaking, do not affect the pharmacological properties of the drug; the second one includes NAb which can decrease and even eliminate the drug's effects.34,35
Binding Ab has been detected in up to 26% of patients treated with alemtuzumab at 12 and at 24 months, with no detrimental effect on the action of the drug. Patients undergoing rituximab treatment develop Abs known as HACA or human antichimeric antibodies. These antibodies appear in 24% of the patients and do not have an impact on the drug's effect, as in cases treated with alemtuzumab. The development of neutralising Abs in patients treated with natalizumab is a completely different case. The phenomenon occurs in 6% of all the patients and is related to the development of side effects, hypersensitivity reactions, and loss of therapeutic effect. In the AFFIRM trial, 57 patients (9%) developed Abs at some point during the study, but only 37 (6%) registered positive on 2 tests taken 42 days apart. These patients showed an increase in reactions to the infusion and experienced a decrease in drug effectiveness.36,37
Development of paradoxical immune responsesmAb are able to promote different autoimmune responses by means of their immunomodulating action. Alemtuzumab is the mAb that has most often been involved in these processes, which are a subject for neurological scrutiny. This drug provoked thrombocytopenia in up to 3% and thyroiditis in up to 22% in a group of MS patients. Another matter of concern for neurologists is the possibility of MS-like demyelinating processes appearing in the CNS in patients treated with mAbs. These processes have been linked most of all to the use of specific mAbs against TNF-α.38
Immune thrombocytopenic purpuraAlemtuzumab is a humanised mAb that targets CD52, a receptor protein that depletes CD4, CD8, natural killer cells, and monocyte cells, while temporarily decreasing levels of B-cells.39,40 This results in thrombocytopenic purpura, which can be fatal. Of the 6 reported cases of ITP, 4 were on doses of 24mg and the other 2 were on doses of 12mg. One case reverted spontaneously, 2 responded well to corticosteroid treatment, and another 2 required rituximab. The last patient's thrombocytopenia persisted despite treatment; in that case, the patient had been treated with interferon beta-1a.23,41
Thrombocytopenia induced by mAb has also been reported in patients treated with infliximab (against TNF-α), efalizumab, and rituximab.
Autoimmune thyroiditisSymptoms of thyroid alteration were recorded in 22% of patients treated with alemtuzumab; 96% of these cases were related to the development of anti-thyroid autoantibodies. Three patients (1.4%) developed severe thyroid alterations; 32 developed hyperthyroidism, which remained chronic in 25 cases, while 6 patients went on to experience persistent hypothyroidism. Four patients required thyroid ablation with radioactive iodine and 24 were treated with long-term antithyroid drugs.23,41 The fact that patients on alemtuzumab treatment had previously undergone treatment with interferon beta may have contributed to the high prevalence of this complication.
Multiple sclerosis-like syndromeAnti-TNF-α antibodies have been linked to the development of antinuclear antibodies and anti-double stranded DNA antibodies. In this context, a lupus-like syndrome may appear. A secondary demyelinating syndrome of the CNS with MS-like demyelinating plaques may also occur, whether or not associated lupus-like symptoms are present.42,43 This syndrome is treated with corticosteroids, and we must be mindful of the fact that its symptoms may appear in a larger context of vasculitis and nephritis. In addition, we must consider that the autoimmune process triggered by these Abs may continue even after mAb treatment has been withdrawn.44
Managing unusual situations during mAb treatmentMononuclear antibody pharmacokinetics are complex and drug effects persist after several cycles of treatment. For example, the rituximab which is initially delivered in 2 cycles 15 days apart remains effective during 6 months, and the alemtuzumab delivered by daily infusions for 5 days remains effective for a year. This leads us to contemplate managing typical situations such as the drug's effect on vaccinations or on pregnancy. In the same way, we will have to face problems derived from withdrawing the drugs (immune reconstitution inflammatory syndrome, IRIS), and we must know how to handle situations in which immunity must be rebuilt because of the appearance of a side effect caused by prolonged immunosuppression.
Vaccination and monoclonal antibodiesRegarding vaccination, we must be aware of the fact that both alemtuzumab and rituximab will cause a loss of the immunity created by vaccines that caused antibodies to develop. In general, live vaccines are formally contraindicated, as they are in patients who have undergone bone marrow transplantation. Attenuated vaccines are safe, and so are the BCG, polio, and typhoid vaccines.
Pregnancy and mAbA recent consensus document recommended withdrawing any type of mAb treatment if the patient becomes pregnant. Experience with this situation is very limited; however, the American College of Rheumatology states that TNF inhibitors may be administered during pregnancy if withdrawing them would place the patient at high risk. In contrast, rituximab and abatacept should be discontinued, since their potential risks for the fetus are unknown. There are currently no studies of other mAbs such as alemtuzumab or daclizumab; the only published evidence regarding natalizumab and gestation was taken from animal studies. Considering the above, it is prudent to recommend that patients use birth control while on mAb treatment, and discontinue treatment in the case of an unplanned pregnancy.45
Monoclonal antibodies in paediatric patientsNatalizumab treatment has been administered to 3 paediatric patients with MS, and their responses were similar to those of adults.46,47 In contrast, there is a large body of evidence about rituximab use in paediatric patients, once again with results comparable to those in adults. No results from paediatric patients are available for any other mAbs (alemtuzumab, daclizumab) used to treat neurological conditions.
Other side effectsApart from the side effects listed previously, quite a few other adverse effects may also appear. These range from haemolytic anaemia induced by alemtuzumab, to DRESS (drug reaction with eosinophilia and systemic symptoms) caused by treatment with daclizumab.48
Immune reconstitution inflammatory syndromeLastly, we should mention a specific phenomenon that may arise spontaneously and can also be induced if so desired. This phenomenon is known as immune reconstitution inflammatory syndrome.49 Current neurological knowledge of this process is based on the neurological deterioration that appeared in AIDS patients who were receiving appropriate courses of highly active antiretroviral therapy (HAART). While this therapy was able to control replication of the HIV virus, it also produced an uncontrolled immune response mediated by CD4 lymphocytes.50 One requirement for IRIS, as established by its definition, is a proven inflammatory component that results from immune recovery and cannot be explained by drug toxicity or appearance of an opportunistic infection.49
IRIS, according to its definition, may occur in the context of 3 different situations which require assessment: (1) appearance of IRIS due to having withdrawn mAb treatment; (2) appearance of IRIS in response to viral seroconversion to a viral infection; and (3) appearance of IRIS in response to hypersecretion of IL-2, the active mechanism in patients undergoing treatment with daclizumab.51
Case-control studies revealed that patients who developed IRIS in cases of JC virus seroconversion had a higher probability of survival.51 Plasmapheresis is indicated in cases of PML in patients treated with natalizumab. The purpose of plasmapheresis is to eliminate the drug from the bloodstream, but it also triggers IRIS, a process which can help control a JC virus infection.
The mechanism by which IRIS occurs is not well-explained. Some sources indicate that IL-2 plays a crucial role in the development of this syndrome, and that an increase in IL-2 levels would result in larger CD4 and CD8 lymphocyte populations.51 In fact, there have been several cases of successful treatment of PML with IL-2 in bone marrow transplant recipients and patients with AIDS.52
The clinical characteristics of IRIS are presence of focal symptoms in conjunction with the appearance of new hyperintense lesions with gadolinium enhancement in MRI studies. Given IRIS and MS flare-up as diagnostic possibilities, differential diagnosis may be impossible. However, whenever inflammatory CNS symptoms appear during or after withdrawal of a mAb, doctors should perform differential diagnosis for IRIS, JC virus seroconversion, and exacerbation of underlying disease. Treatment consists of intravenous methylprednisolone dosed at 1g per month over 6 months.53
ConclusionsMonoclonal antibodies constitute a very effective drug group, but they are also associated with side effects which may be quite serious (Table 6). From a practical point of view, patients must be selected correctly, treatments must be delivered in an outpatient clinic by trained personnel, and the clinic must be equipped to respond in the event of cardiorespiratory failure. With natalizumab therapy, doctors recommend monitoring the patient for an hour after treatment has been administered. Each unit or clinic should have specific treatment initiation and monitoring protocols for every mAb being delivered.
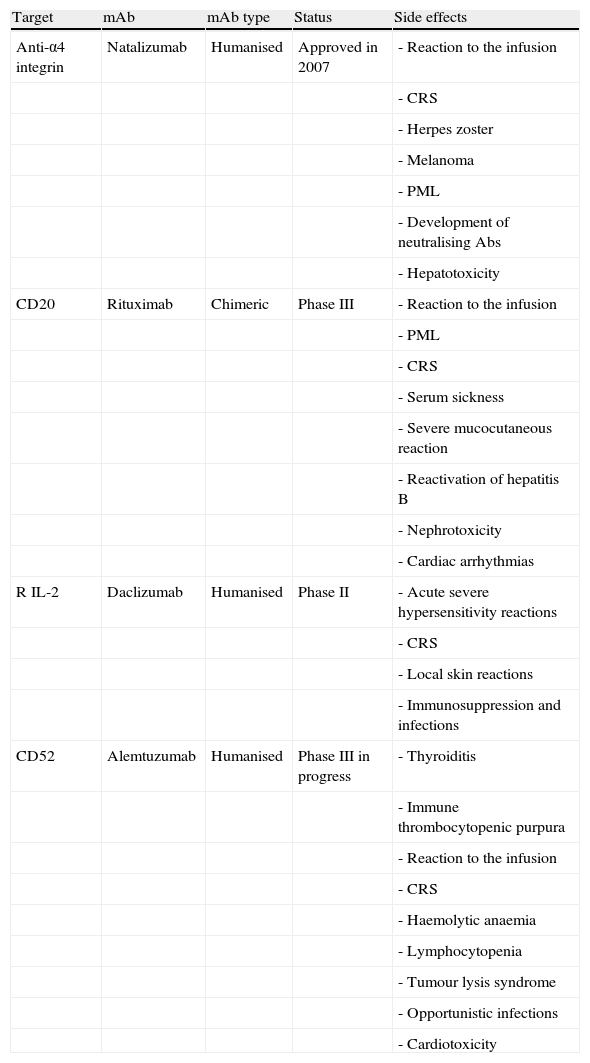
Summary of the most frequent neurological side effects of mAbs according to completed phase II clinical trials.
| Target | mAb | mAb type | Status | Side effects |
| Anti-α4 integrin | Natalizumab | Humanised | Approved in 2007 | - Reaction to the infusion |
| - CRS | ||||
| - Herpes zoster | ||||
| - Melanoma | ||||
| - PML | ||||
| - Development of neutralising Abs | ||||
| - Hepatotoxicity | ||||
| CD20 | Rituximab | Chimeric | Phase III | - Reaction to the infusion |
| - PML | ||||
| - CRS | ||||
| - Serum sickness | ||||
| - Severe mucocutaneous reaction | ||||
| - Reactivation of hepatitis B | ||||
| - Nephrotoxicity | ||||
| - Cardiac arrhythmias | ||||
| R IL-2 | Daclizumab | Humanised | Phase II | - Acute severe hypersensitivity reactions |
| - CRS | ||||
| - Local skin reactions | ||||
| - Immunosuppression and infections | ||||
| CD52 | Alemtuzumab | Humanised | Phase III in progress | - Thyroiditis |
| - Immune thrombocytopenic purpura | ||||
| - Reaction to the infusion | ||||
| - CRS | ||||
| - Haemolytic anaemia | ||||
| - Lymphocytopenia | ||||
| - Tumour lysis syndrome | ||||
| - Opportunistic infections | ||||
| - Cardiotoxicity |
PML: progressive multifocal leucoencephalopathy; CRS: cytokine release syndrome.
Lastly, the rates of serious adverse reactions are as follows: 10% for reactions to rituximab infusion; 22% for autoimmune reactions to alemtuzumab; and 0.15% for PML with natalizumab treatment. In addition to their being highly effective, these drugs also have a very good safety profile as a result of the risk prevention measures that have been implemented in both the European Union and the United States.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Casanova Estruch B. Perfil de seguridad y aspectos prácticos a tener en cuenta en la administración de anticuerpos monoclonales. Neurología. 2013;28:169–78.