The increased morbidity and mortality and poorer quality of life associated with drug-resistant epilepsy justify admitting patients to epilepsy monitoring units (EMU). These units employ methods that promote the occurrence of seizures, which involves a risk of secondary adverse events. The aim of our study was to characterise and quantify these adverse events in a Spanish EMU.
Materials and methodsA descriptive, longitudinal and retrospective study of patients admitted consecutively to our EMU. Patients admitted due to status epilepticus, clusters of seizures, or as participants in a clinical trial were excluded.
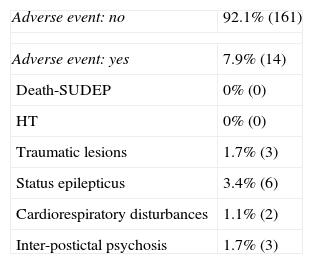
ResultsWe included 175 patients, of whom 92.1% (161) did not suffer any adverse events. Status epilepticus was present in 3.4% (6), 1.7% (3) had traumatic injury, 1.7% (3) had interictal or postictal psychosis, and 1.1% (2) had cardiorespiratory impairment. There were no risk factors associated with these adverse events.
ConclusionsThe most frequently identified adverse events were status epilepticus, traumatic injury, interictal or postictal psychosis, and cardiorespiratory disorders. The frequency of these adverse events was similar to that seen in the international literature. The complications detected do not contraindicate VEEGM.
En epilepsia farmacorresistente el incremento asociado de la morbimortalidad y el deterioro de calidad de vida hace necesario el ingreso en Unidades de Monitorización de Epilepsia (UME). En dichas Unidades se practican técnicas que facilitan la aparición de crisis epilépticas, implicando un riesgo de aparición de fenómenos adversos secundarios. El objetivo de nuestro estudio es caracterizar y cuantificar dichos fenómenos adversos en una UME en España.
Materiales y métodosEstudio descriptivo, longitudinal y retrospectivo de pacientes consecutivos ingresados en nuestra UME. Se excluyó a los pacientes que ingresaron por motivo de status epilepticus, serie de crisis o ensayo clínico.
ResultadosSe incluyeron 175 pacientes. Un 92,1% (161) de los pacientes no presentó ningún fenómeno adverso. Un 3,4% (6) presentó status epilepticus, un 1,7% (3) presentó lesión traumática, un 1,7% (3) presentó alteración psiquiátrica inter-postictal, un 1,1% (2) presentó alteración cardiorrespiratoria de riesgo. No se detectaron factores de riesgo asociados a dichos fenómenos adversos.
ConclusionesLos fenómenos adversos detectados con mayor frecuencia fueron el status epilepticus, lesiones traumáticas, alteraciones psiquiátricas inter-postictales y alteraciones cardiorrespiratorias. La frecuencia de aparición de dichos fenómenos adversos fue similar al de series internacionales. Las complicaciones detectadas no contraindican la MVEEG.
Experience with the diagnosis and treatment of epileptic patients shows that approximately one-third of all patients are resistant to pharmacological treatment.1 Mortality in this group is 2 to 3 times higher than that in the general population.2,3
Admitting these patients to an epilepsy monitoring unit (EMU) is justified due to their lower life expectancy4 and the presence of complications such as sudden unexpected death in epilepsy (SUDEP),5 status epilepticus, accidents, and suicide. Admission to an EMU is fundamental for pre-surgical and differential diagnoses of drug-resistant epileptic patients.6,7 In these units, professionals systematically apply techniques intended to trigger seizures. As a result, seizure-related adverse events are also more likely to appear.8–10
Recent studies indicate an adverse event rate of 11%. The most frequently observed events included postictal psychosis (1.3%–3.9%), traumatic lesions (2.75%–2.95%), status epilepticus (0.67%–1.97%), and high-risk autonomic alterations (2%).11,12
This study aims to describe seizure-related adverse events during video-EEG monitoring (VEEGM), identify those patients with the highest risk of presenting one or more such events, and determine which procedures entail the greatest risk. To our knowledge, this is the first study in the Spanish literature to analyse safety during VEEGM, which may be an important consideration for the elaboration of standards and action protocols.
Materials and methodsThis descriptive longitudinal retrospective study analysed the medical history and records of all patients consecutively admitted to the EMU between December 2009 and July 2012 (31 months). Patients admitted to the UME for pre-surgical or differential diagnosis were included in the study. Exclusion criteria were having undergone video-EEG monitoring as part of a clinical study or because of status epilepticus or serial seizures. We documented all events occurring during hospitalisation and analysed seizure-related adverse events with regard to physical safety during VEEGM. The main events selected for analysis were death, myoclonic status epilepticus (MSE), seizure-related traumatic lesions, status epilepticus (SE), high-risk cardiorespiratory changes, and interictal or postictal psychosis. VEEGM was indicated by a team of epilepsy specialists.
Patients were referred by outpatient consults in our centre and by other Catalan or Spanish centres. VEEGM was generally performed over 5 days, but the duration was modified according to the patient's needs.
At the time of admission, doctors recorded the patient's personal history, performed a physical examination, and completed a blood test. All patients underwent a 3T MRI scan according to the epilepsy protocol and most also completed a neuropsychological study. All subjects gave their informed consent. Researchers then placed electrodes according to the international 10-20 or 10-10 system, depending on each case. Sphenoidal electrodes were used in certain patients suspected of having temporal lobe seizures. Patients were assessed in protected and adapted beds with continuous video-EEG monitoring 24hours a day over 5 weekdays; during this time, they were monitored by specialised technical staff.
Prophylactic anticoagulant treatment was indicated in patients with vascular risk factors or those older than 50 years. Researchers registered and analysed all seizures and adverse events. Walking was restricted during VEEGM. A group of neurologists, nurses, and technical personnel with specific training in epilepsy monitored events experienced by each patient over each full 24-hour period.
Each patient's individual situation was considered in decisions to withdraw AEDs, indicate sleep deprivation, apply the flumazenil test, or induce non-epileptic seizures. Decisions to perform an ictal SPECT study and other diagnostic procedures were also made on a case-by-case basis.
The method employed when discontinuing AEDs was based on each patient's previous seizure frequency, drug half-life, the possibility of seizures due to treatment withdrawal, and history of SE or serial seizures. The flumazenil test was only applied to patients whose epileptic seizures did not appear spontaneously after AEDs were discontinued.13 Non-epileptic seizures were induced in patients suspected of having this condition in cases in which seizures did not spontaneously appear during the first days of VEEGM and after complete withdrawal of AEDs. The test was performed according to the Bonn University method and approved by our hospital's bioethics committee.
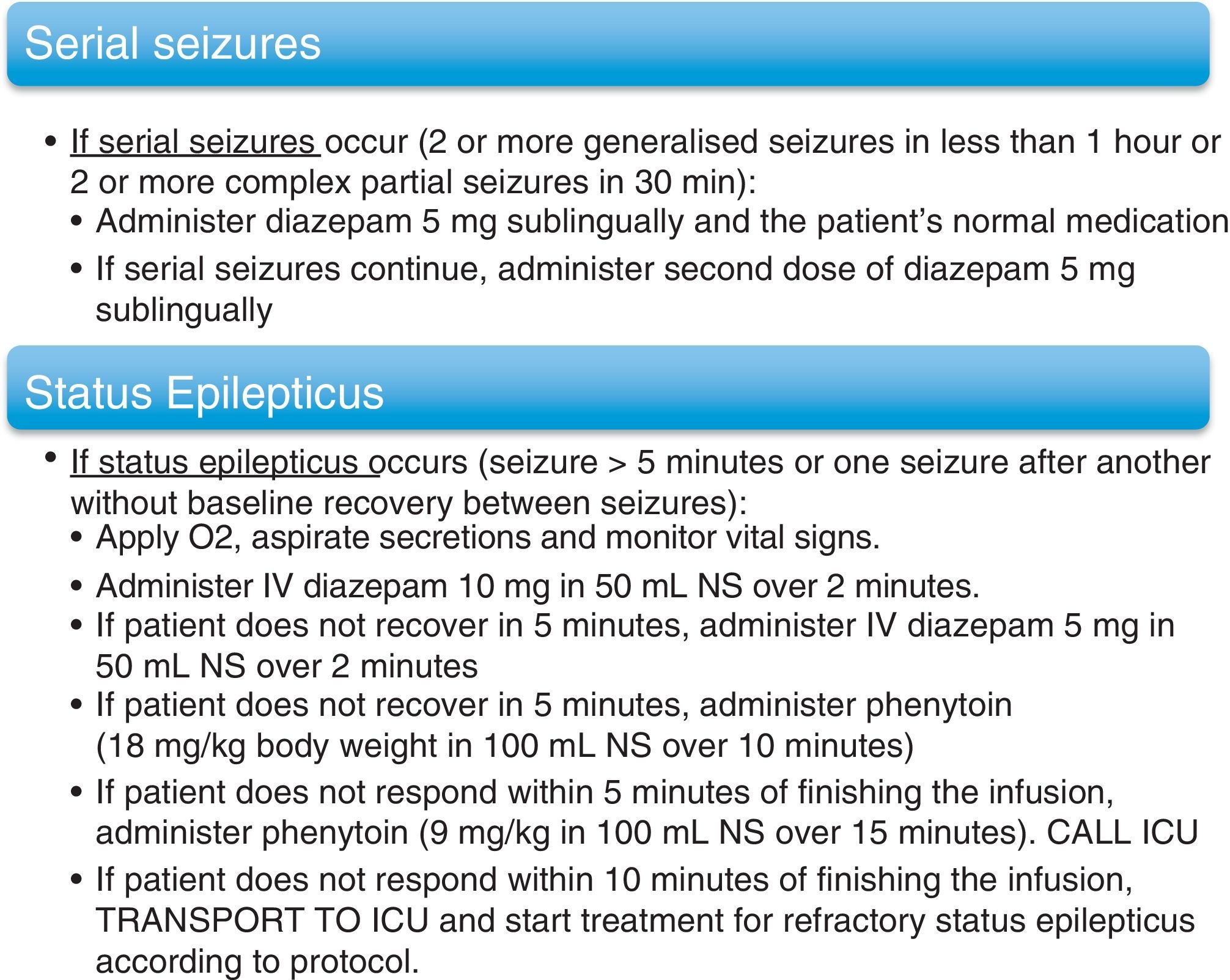
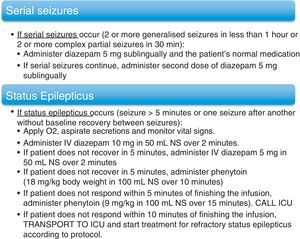
In cases in which invasive monitoring was indicated, researchers implanted subdural electrodes. Special bandaging protocols were followed for patients fitted with these electrodes. Action protocols were applied in cases of serial seizures and SE. These protocols are listed in (Fig. 1). Once the VEEGM study was complete, patients resumed AED treatment, which was modified according to individual need and the data gathered during monitoring.
Since the variables ‘age’ and ‘length of stay in the EMU’ displayed a normal distribution, we used the t-test for intragroup comparison. For other variables without a normal distribution, we used the Spearman rank correlation coefficient to determine the correlation between the quantitative variables ‘number of seizures per month before admission’ and ‘number of seizures during hospitalisation’. The Mann–Whitney U test was used for intragroup comparison of continuous variables (‘duration of epilepsy prior to admission’ and ‘number of seizures during hospitalisation’). The chi-square test was employed for qualitative variables (sex, history of SE or psychiatric disease, discontinuation of AEDs, sleep deprivation, and ictal SPECT study). We employed the paired-sample t-test to compare seizure frequency between the month prior to and the month after hospitalisation.
Univariate and multivariate regression analyses were used to identify independent risk factors for adverse events, including demographic data (age, sex) and clinical data (epilepsy duration, reason for admission, history of SE, decreases in AED dosage, sleep deprivation, performing ictal SPECT test, seizure induction/provocation tests). Due to the low number of adverse events detected in the study, we created the umbrella category ‘adverse event’ which included SE variables, traumatic lesions, high-risk cardiorespiratory disturbances, and seizure-related psychiatric complications.
Lastly, we performed logistic regression analyses with the potential risk factors for adverse events. These analyses showed statistically significant or near-significant correlations in the univariate analysis.
ResultsWe analysed 301 video-EEG monitoring sessions from 236 patients. A total of 175 patients and 220 monitoring sessions were included in the study according to the inclusion and exclusion criteria. Sixty-one patients and 81 monitoring sessions were excluded because of meeting the exclusion criteria.
One hundred and thirty-five patients (77.1%) had a single monitoring session; 35 had two sessions (20%) and 5 required 3 sessions (2.9%). None of the patients needed more than 3 monitoring tests. Of the patient total, 32% (32) were admitted for differential diagnosis and 68% (119) of the patients were admitted for a pre-surgical diagnosis. The total number of days in hospital was 1013.
The number of seizures before admission was significantly correlated to the number of seizures during hospitalisation, with a correlation coefficient of 0.442 (P<.001). Tables 1–3 show sample descriptions, occurrences during each session, and any adverse events observed.
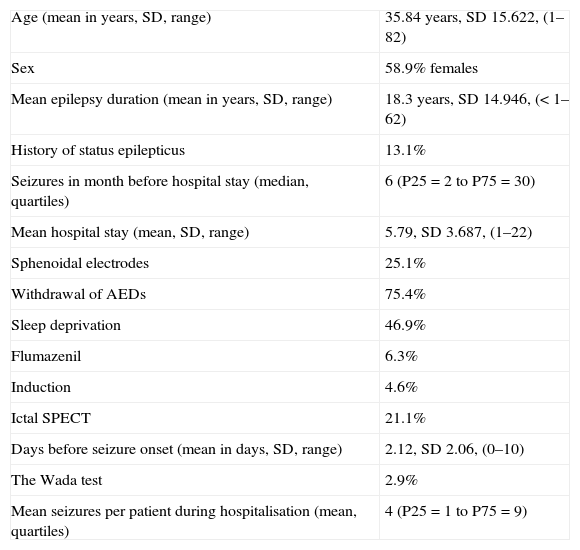
Demographic data.
| Age (mean in years, SD, range) | 35.84 years, SD 15.622, (1–82) |
| Sex | 58.9% females |
| Mean epilepsy duration (mean in years, SD, range) | 18.3 years, SD 14.946, (<1–62) |
| History of status epilepticus | 13.1% |
| Seizures in month before hospital stay (median, quartiles) | 6 (P25=2 to P75=30) |
| Mean hospital stay (mean, SD, range) | 5.79, SD 3.687, (1–22) |
| Sphenoidal electrodes | 25.1% |
| Withdrawal of AEDs | 75.4% |
| Sleep deprivation | 46.9% |
| Flumazenil | 6.3% |
| Induction | 4.6% |
| Ictal SPECT | 21.1% |
| Days before seizure onset (mean in days, SD, range) | 2.12, SD 2.06, (0–10) |
| The Wada test | 2.9% |
| Mean seizures per patient during hospitalisation (mean, quartiles) | 4 (P25=1 to P75=9) |
SD: standard deviation; AED: antiepileptic drug.
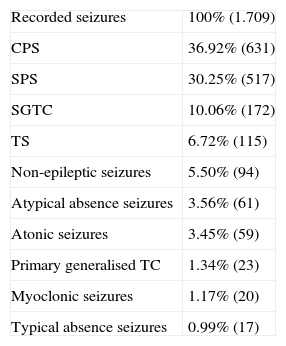
Recorded seizures.
| Recorded seizures | 100% (1.709) |
| CPS | 36.92% (631) |
| SPS | 30.25% (517) |
| SGTC | 10.06% (172) |
| TS | 6.72% (115) |
| Non-epileptic seizures | 5.50% (94) |
| Atypical absence seizures | 3.56% (61) |
| Atonic seizures | 3.45% (59) |
| Primary generalised TC | 1.34% (23) |
| Myoclonic seizures | 1.17% (20) |
| Typical absence seizures | 0.99% (17) |
SGTC: secondary generalised tonic–clonic seizures; CPS: complex partial seizures; SPS: simple partial seizures; TS: tonic seizure; TC: tonic–clonic.
Complications during VEEGM.
| Adverse event: no | 92.1% (161) |
| Adverse event: yes | 7.9% (14) |
| Death-SUDEP | 0% (0) |
| HT | 0% (0) |
| Traumatic lesions | 1.7% (3) |
| Status epilepticus | 3.4% (6) |
| Cardiorespiratory disturbances | 1.1% (2) |
| Inter-postictal psychosis | 1.7% (3) |
SUDEP: sudden unexpected death in epilepsy; HT: head trauma.
All cases of SE responded favourably to treatment in the EMU except for one patient who had to be admitted to the ICU because of meeting criteria for refractory SE. There were no complications and the patient later resumed monitoring.
Patients presented no severe traumatic lesions, except for one case of double vertebral compression fracture, one spinal disc herniation, and one relapse in a case of chronic glenohumeral luxation. One patient suffered pronounced tachycardia (HR<200) and another experienced severe postictal hypopnea with prolonged flatline on the EEG following the seizure. Three patients experienced postictal psychiatric alterations (2 cases of postictal psychosis and 1 case of postictal depression).
Patients were asked to remain in contact with the epilepsy team to confirm the presence or absence of these complications after hospitalisation. Overall, 7.9% of the patients presented a seizure-related adverse event during video-EEG monitoring; 5.1% of the cases were severe (SE, vertebral fracture, spinal disc herniation, and hypopnea). Only 2 adverse events (1.14%: vertebral fracture, spinal disc herniation) still had consequences after discharge from hospital.
To examine risk or protective factors for seizure-related adverse events, we analysed the following variables: sex, age, epilepsy duration, history of SE, history of psychiatric disease, withdrawal of AEDs, sleep deprivation, length of stay in the EMU, number of seizures during hospitalisation, type of predominant seizure, use of ictal SPECT test, use of flumazenil test, and induction of non-epileptic seizures. Our series did not identify risk or protective factors that would affect the probability of seizure-related adverse events. The variables ‘withdrawal of AEDs’ and ‘sleep deprivation’ were inversely related to the number of days before onset of the first seizure (P=.035 and P=.001, respectively). We observed a statistically significant relationship between withdrawing treatment and number of seizures during the hospital stay (P=.004).
Seventeen patients were diagnosed with non-epileptic seizures and 5 were diagnosed with diseases other than epilepsy (concomitant epilepsy was ruled out). An electrocorticography study with subdural electrodes or sEEG was indicated in 13 patients (10.92% of the total pre-surgical study group). Three patients had already undergone this procedure by the end of our study period. Of the 119 patients admitted for pre-surgical diagnosis, epilepsy surgery was indicated in 51 (42.8% of the total). Within this group, 27 patients have already undergone surgery and 24 are still pending surgery. Surgery was indicated in order to implant a device for vagus nerve stimulation (VNS) in 14 patients, of which 6 had already undergone this procedure by the end of our study period.
DiscussionThere were no cases of death in this study, or in other similar series.11,12 However, myoclonic status epilepticus (MSE) has been reported as a complication during VEEGM.14
Regarding morbidity, 7.9% of our patients presented adverse events. That percentage was 9% in the study by Trinka et al. (44 of 507 patients); however, some patients presented multiple adverse events (53 events in 507 patients). Therefore, the differences in percentage between their results and our own could be explained by the different sample sizes (175<507) and differences in the reporting of adverse events (death, traumatic lesions, SE, seizure-related psychiatric complications, and adverse drug effects; high-risk autonomic alterations were not reported here).
The study by Noe and Drazkowski showed a 14% rate of adverse events; however, this study applied more restrictive inclusion criteria given that only patients with tonic–clonic seizures were included in the study (patients who presented absence seizures, non-epileptic seizures, etc. were excluded).
The study by Trinka et al. reported 15 patients presenting a total of 19 traumatic lesions (14 lesions caused by falls, 2 fractures caused by falls, 2 fractures without falling, and one case of head trauma with epidural intracranial haemorrhage). Our series presents a lower percentage of severe traumatic lesions (1.14%) with only one vertebral fracture and one spinal disc herniation. This difference was most likely due to the fact that walking was restricted during VEEGM in our EMU. Similarly, Noe and Drazkowski indicated that traumatic lesions were present in 2.75% of their group, in which 4 patients presented compression fractures of the vertebrae following seizures with secondary generalisation. No cases of death secondary to traumatic lesion were reported in any of the series.
Compared to our series, in which SE was present in 3.4% of the total, the study by Trinka et al. reported SE in 2.5% of their group (13 episodes in 507 patients). The same definition of SE was used in both studies (seizure lasting <5–10minutes). In the study by Noe and Drazkowski, 0.67% of the patients presented SE (1 case), but their definition of SE required a duration of 30minutes. These episodes should be defined as prolonged seizures (2 cases) in order for the data to be comparable. As in our series, neither of the 2 studies described above reported the need for endotracheal intubation or anaesthetic care to treat SE.
Our series detected 2 cases of high-risk cardiorespiratory changes (1.1%). Whereas such changes were not studied by Trinka et al., Noe and Drazkowski detected 3 cases in 149 patients (2%). Our series found that 1.7% of the patients (3 cases) had inter-postictal psychiatric disorders; the percentage reported by Trinka et al. was 3.9% (20 cases in 507 patients: postictal psychosis, interictal psychosis, and panic attacks). Noe and Drazkowski reported a rate of 1.34% (2 cases in 149 patients, with postictal psychosis being the only psychiatric disturbance). Contrary to results from other studies, inter-postictal psychosis was a rare event in our series. This result is probably related to the more restrictive psychiatric exclusion criteria we applied when selecting candidates for VEEGM. Given that walking was not allowed in our EMU for safety reasons, it was necessary to select patients who would be able to tolerate this restriction. Our series showed a significant decrease in patients’ mean seizure frequency before and after being hospitalised in the EMU, which confirms that VEEGM is an effective means of reducing seizure frequency in patients with drug-resistant epilepsy. As was expected, the decrease in seizure frequency was much larger in patients who were treated surgically. Furthermore, being included in this study resulted in some patients being assigned a different diagnosis. As a result, their treatment approaches could be optimised after hospitalisation. Treatment with AEDs was suspended in patients diagnosed with non-epileptic seizures or with other non-epileptic crises. Considering these benefits, the adverse events that occurred in our series did not contraindicate admitting patients to the EMU.
In conclusion, the overall frequency of seizure-related adverse events during VEEGM in our study resembles figures from larger international series. The adverse events we recorded were not severe enough to contraindicate VEEGM considering the risks inherent to epilepsy. Only 1.14% of the patients had complications whose sequelae were still present after discharge from hospital. Although our study detected no cases of SUDEP, this event may occur in patients who are already at risk for this complication, which has been reported in the international literature during VEEGM.14 In light of these results, monitoring patients in an EMU while following appropriate protocols is a safe method for determining differential and pre-surgical diagnoses for patients with drug-resistant epilepsy.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ley M, Vivanco R, Massot A, Jiménez J, Roquer J, Rocamora R. Estudio de seguridad en la monitorización por vídeo-electroencefalograma prolongado. Neurología. 2014;29:21–26.