This study addresses the survival of consecutive patients with high-grade gliomas (HGG) treated at the same institution over a period of 10 years. We analyse the importance of associated factors and the role of salvage surgery at the time of progression.
MethodsWe retrospectively analysed a series of patients with World Health Organization (WHO) grade III/IV gliomas treated between 2008 and 2017 at Hospital Gregorio Marañón (Madrid, Spain). Clinical, radiological, and anatomical pathology data were obtained from patient clinical histories.
ResultsFollow-up was completed in 233 patients with HGG. Mean age was 62.2 years. The median survival time was 15.4 months. Of 133 patients (59.6%) who had undergone surgery at the time of diagnosis, 43 (32.3%) underwent salvage surgery at the time of progression. This subgroup presented longer overall survival and survival after progression. Higher Karnofsky Performance Status score at diagnosis, a greater extent of surgical resection, and initial diagnosis of WHO grade III glioma were also associated with longer survival.
ConclusionsAbout one-third of patients with HGG may be eligible for salvage surgery at the time of progression. Salvage surgery in this subgroup of patients was significantly associated with longer survival.
Analizar la supervivencia en el grupo de pacientes con gliomas de alto grado tratados de forma consecutiva en un mismo centro a lo largo de diez años. Establecer la relevancia de los factores asociados y el papel de la cirugía de rescate en el momento de la progresión.
MetodosFueron analizados de forma retrospectiva los pacientes con gliomas grado III y IV de la Organización Mundial de la Salud (OMS) diagnosticados en el Hospital Gregorio Marañón desde el 1 de Enero de 2008 al 31 de Diciembre de 2017. Se obtuvieron de la historia clínica los datos clínicos, radiológicos y anatomopatológicos.
ResultadosSe completó el seguimiento en 233 pacientes con diagnóstico de glioma de alto grado (III o IV de la OMS). La edad media fue de 62,2 años. La mediana de supervivencia se situó en 15,4 meses. De los 133 pacientes (59,6%) que habían sido intervenidos mediante cirugía resectiva en el momento del diagnóstico, en 43 (32,3%) se llevó a cabo cirugía de rescate en el momento de la progresión. La supervivencia global, así como la supervivencia tras la progresión resultó mayor en este subgrupo de pacientes. Otras variables relacionadas con una mayor supervivencia fueron la puntuación en la escala de Karnofsky (KPS), el grado de resección quirúrgica (GR) y el diagnóstico inicial de grado III de la OMS.
ConclusionesAlrededor de una tercera parte de los pacientes con gliomas de alto grado pueden ser candidatos a una cirugía de rescate en el momento de la progresión. Ello está asociado a una mayor supervivencia.
The treatment of patients with high-grade glioma remains one of the most dispiriting areas in neurosurgical practice. While new therapeutic tools have appeared in recent years, their benefits in terms of survival and quality of life have generally been modest. In fact, years ago, the great aggressiveness and fast progression of the disease led to significant questioning of therapeutic efforts, in the light of their lack of effectiveness.1
While there is currently consensus on the management of newly diagnosed high-grade glioma, there is no such agreement regarding how to proceed in patients presenting recurrence after a first-line treatment.2 Recurrence is largely inevitable, given the biological behaviour of these tumours. The heterogeneity of patients with recurrent high-grade glioma, in terms of their general status at the time of relapse, the extent of resection (EOR) in the initial procedure, and the anatomical regions involved, constitutes a significant challenge for interpreting the results of studies in this group of patients.2 However, it is thought that 20%–30% of patients with recurrent glioblastoma may benefit from salvage surgery.3
There is currently considerable interest in determining the value or futility of treatment efforts of similar intensity to those used at the time of initial diagnosis and identifying patient profiles most likely to benefit from such treatments.1,4
MethodsWe conducted a retrospective study of adult patients with glial tumours of astrocytic lineage, supratentorial localisation, and histologic grade III–IV according to the World Health Organization (WHO) classification, diagnosed at Hospital General Universitario Gregorio Marañón over a 10-year period from January 2008 to December 2017. We conducted non-probability sampling of consecutive patients, who were included in the order of intervention. Data were gathered from the records of the hospital’s Neuro-Oncology Committee. We excluded patients with histology findings of ependymoma, ganglioglioma, oligodendroglioma, anaplastic oligoastrocytoma, astroblastoma, chordoid glioma of the third ventricle, or angiocentric glioma. We also excluded all patients younger than 18 years.
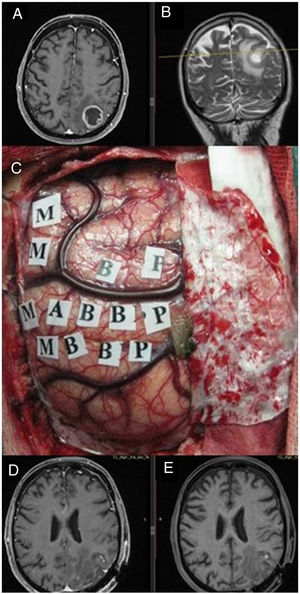
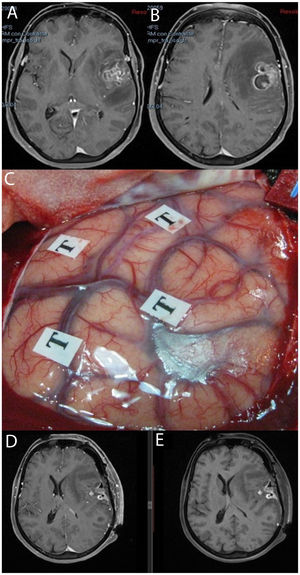
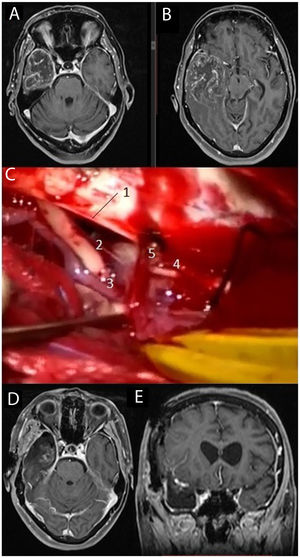
Data were collected on age, sex, date of diagnosis, Karnofsky Performance Status (KPS) score, therapeutic approach (with or without surgery; type of surgery), progression-free survival after the initial surgical procedure, number of interventions, type of adjuvant treatment, and cause of death. We also referred to radiology images and reports to obtain data on tumour involvement of eloquent regions, defined as extension to the thalamocapsular area, corona radiata, centrum semiovale, central lobule, or language-related areas (frontal operculum of the dominant hemisphere, supramarginal gyrus, dominant temporal lobe, and connections between these areas). Resection of tumours in these locations was assisted by intraoperative neurophysiological monitoring (Fig. 1) or was performed with the patient awake (Fig. 2) in order to maximise the EOR and limit the risk of postoperative neurological deficits (Fig. 3).
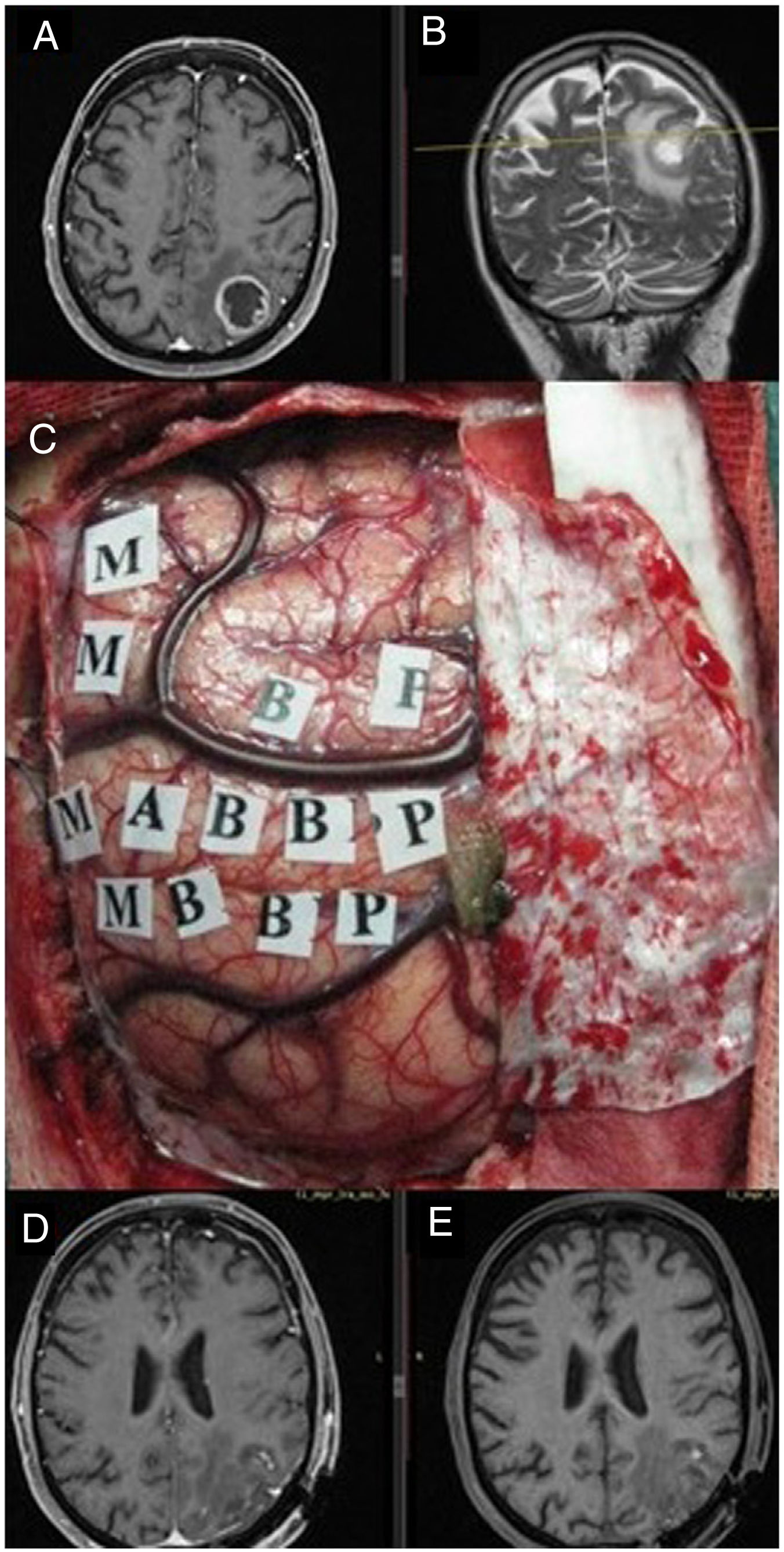
MRI study: post-contrast axial T1-weighted sequence (A) and coronal T2-weighted sequence (B) showing a tumour corresponding to glioblastoma, located at the posterior edge of the left parietal central lobe. We performed surgery with intraoperative brain mapping to identify the motor cortex (C), enabling complete resection of the tumour, as shown in the T1-weighted sequence. Postoperative MRI study with and without intravenous contrast (D and E, respectively), with no postoperative neurological deficits.
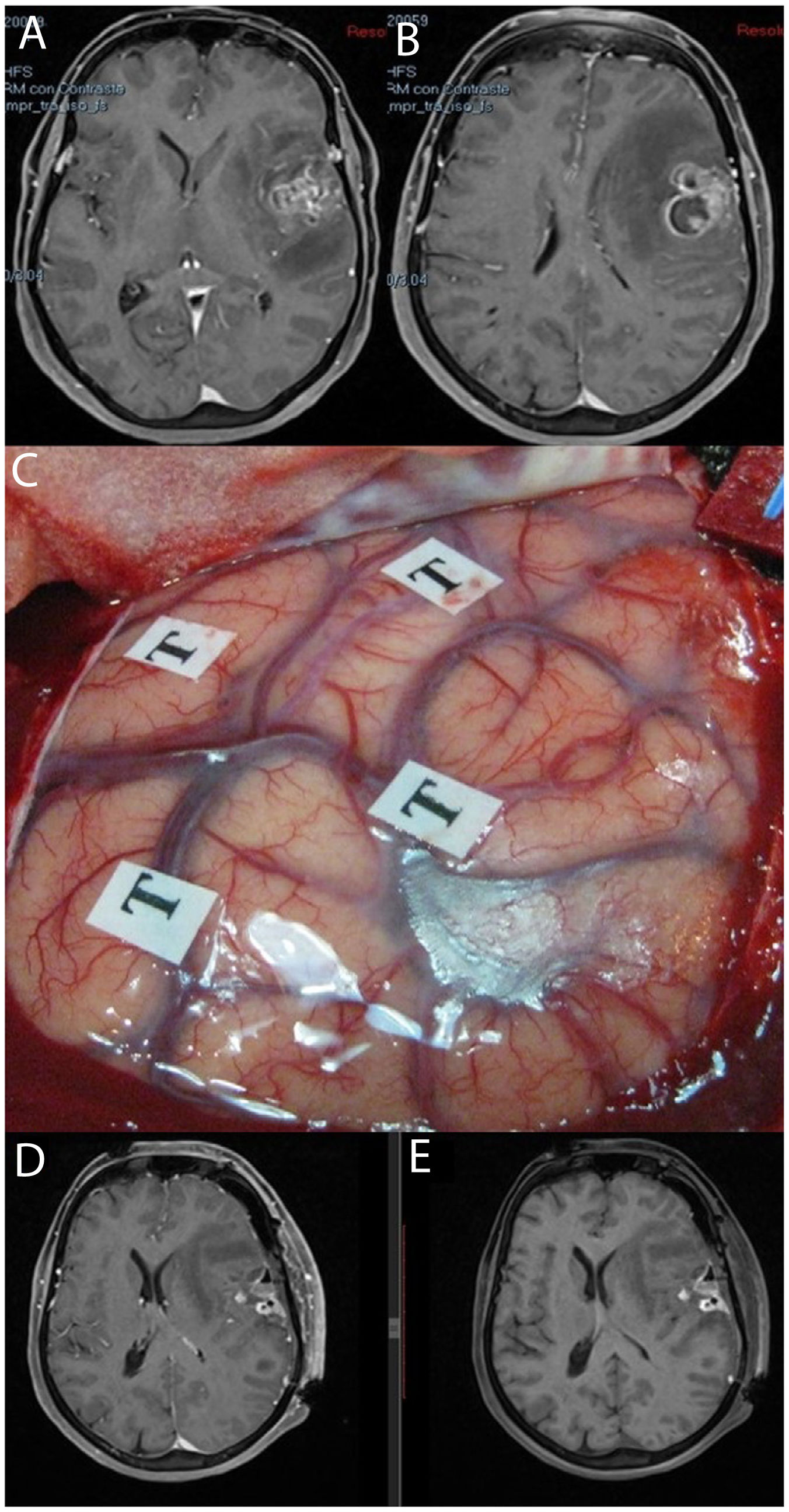
MRI study: post-contrast T1-weighted sequences (A and B) showing a tumour corresponding to glioblastoma located in the left opercular region. We performed awake surgery with intraoperative brain mapping (C). Postoperative MRI sequences with and without intravenous contrast (D and E, respectively) showed postoperative changes, with blood residue in the absence of residual tumour; the patient displayed no worsening of language.
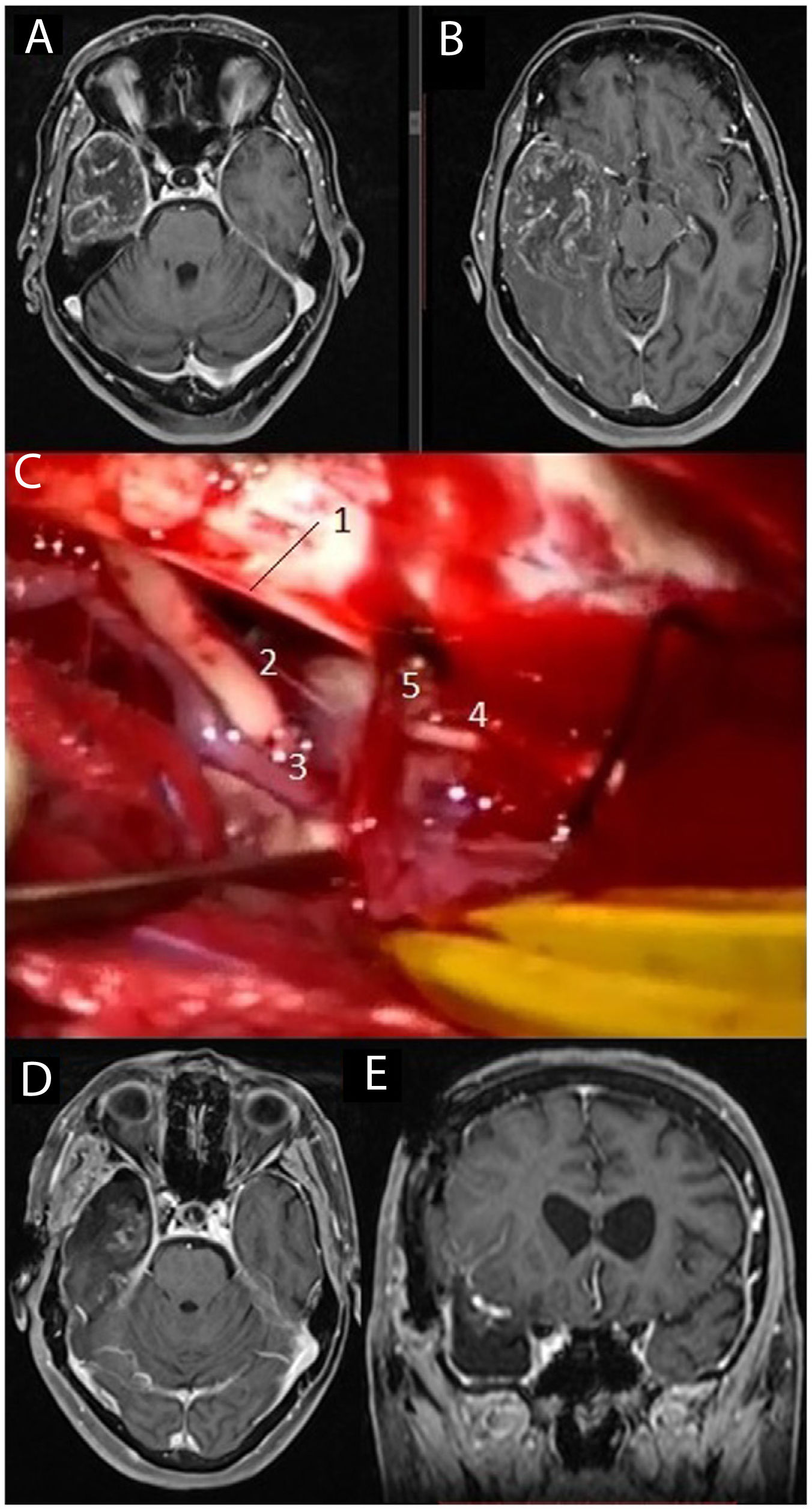
MRI study: post-contrast T1-weighted sequences (A and B) showing a large tumour corresponding to glioblastoma located in the right temporal lobe. We performed temporal lobectomy; the intraoperative image (C) shows the tentorium cerebelli free border (1), the right third cranial nerve (2), the right posterior communicating artery (3), and the right fourth cranial nerve (4) alongside a cortical vein draining into the tentorium (5). Postoperative axial and coronal MRI sequences (D and E, respectively).
The EOR in the initial procedure (EOR1) was determined in an MRI study conducted in the first 72 h after surgery (Figs. 1–3), and classified according to the criteria proposed by Bloch et al.5 as subtotal (≤ 95% of the contrast-enhancing region) or gross-total (> 95% of the contrast-enhancing region).
Histopathological characterisation of the tumour was based on the 2007 WHO classification.6 Tumour grade was established according to the WHO criteria at the time of diagnosis and, where applicable, at the time of salvage surgery.
Adjuvant treatment was based on the protocol described in 2005 by Stupp et al.,7 with 60 Gy of radiation administered in 30 sessions of 2 Gy each over 6 weeks (5 sessions per week), with concomitant administration of temozolomide 7 days per week from the first to the last day of radiotherapy. This was followed by 6 cycles of treatment with temozolomide; each cycle lasted 28 days, with 5 consecutive days of drug administration and 23 days of rest.7
Patients were followed up until 31 December 2018 by the neurosurgery, medical oncology, and radiotherapeutic oncology departments. As part of this follow-up, patients underwent brain MRI studies every 3 months. Progression-free survival was defined as the time from treatment onset until the detection of tumour recurrence according to the Response Assessment in Neuro-Oncology (RANO) criteria.8 Recurrence may be observed in routine follow-up MRI studies or in imaging studies requested in response to neurological worsening.
Indication of salvage surgery was established at the time of recurrence on a case-by-case basis, based on consensus of the neuro-oncology committee. The committee was made up of specialist physicians involved in the treatment of the disease: neurosurgeons, medical oncologists, and radiotherapeutic oncologists, as well as radiodiagnosis and anatomical pathology specialists. It also included administrative staff responsible for recording the decisions made in each case. Each case was discussed individually in order to make choices based on scientific rigour and the particular situation of each patient, reaching a binding, consensus-based treatment decision.
Salvage surgery was indicated at the time of recurrence, according to the following criteria:
- •
KPS > 70
- •
Progression-free survival longer than 6 months
- •
Favourable tumour location enabling at least subtotal resection without risk of disabling neurological deficits.
Kaplan–Meier curves were used for survival analysis. The impact of each variable on survival was calculated using the log-rank test. Cox regression was used to calculate the risk stratification of variables displaying a significant impact. The threshold for statistical significance was set at P < .05. Statistical analysis was conducted with the SPSS statistics software, version 19.0 (IBM; New York, United States).
ResultsBetween January 2008 and December 2017, we identified a total of 328 patients diagnosed with grade III or IV glial tumour according to the WHO criteria: 185 men (54.3%) and 143 women (43.6%).
Follow-up was completed in 223 patients (68%), with a mean (standard deviation [SD]) follow-up period of 14.3 (10.9) months. The mean age of patients in the sample was 62.21 (13.89) years. Baseline KPS score was 100 in 90 patients (40.3%), 90 in 49 (22%), 80 in 72 (32.3%), 70 in 7 (3.1%), and 60 or less in 5 (2.2%).
Tumours were located in non-eloquent areas in 150 patients (67.3%) and in eloquent areas in 73 (32.7%). A total of 133 patients (59.6%) underwent resective surgery at the time of diagnosis, with 70 (31.4%) undergoing diagnostic surgery (navigation-guided or stereotactic excisional biopsy); surgery was ruled out in 20 cases (9%). With respect to EOR, gross-total resection was possible in 89 cases (66.9%), whereas resection was subtotal in the remaining 44 (33.1%). According to the WHO histological classification, tumours were grade III in 38 patients (18.7%) and grade IV in 165 (81.3%). The median progression-free survival time recorded was 10.9 months (1.0–34.8).
At the time of recurrence after first-line treatment, salvage surgery was performed in 43 of the 133 patients (32.3%) who had initially undergone resective surgery, in order to reduce tumour load to the greatest possible extent.
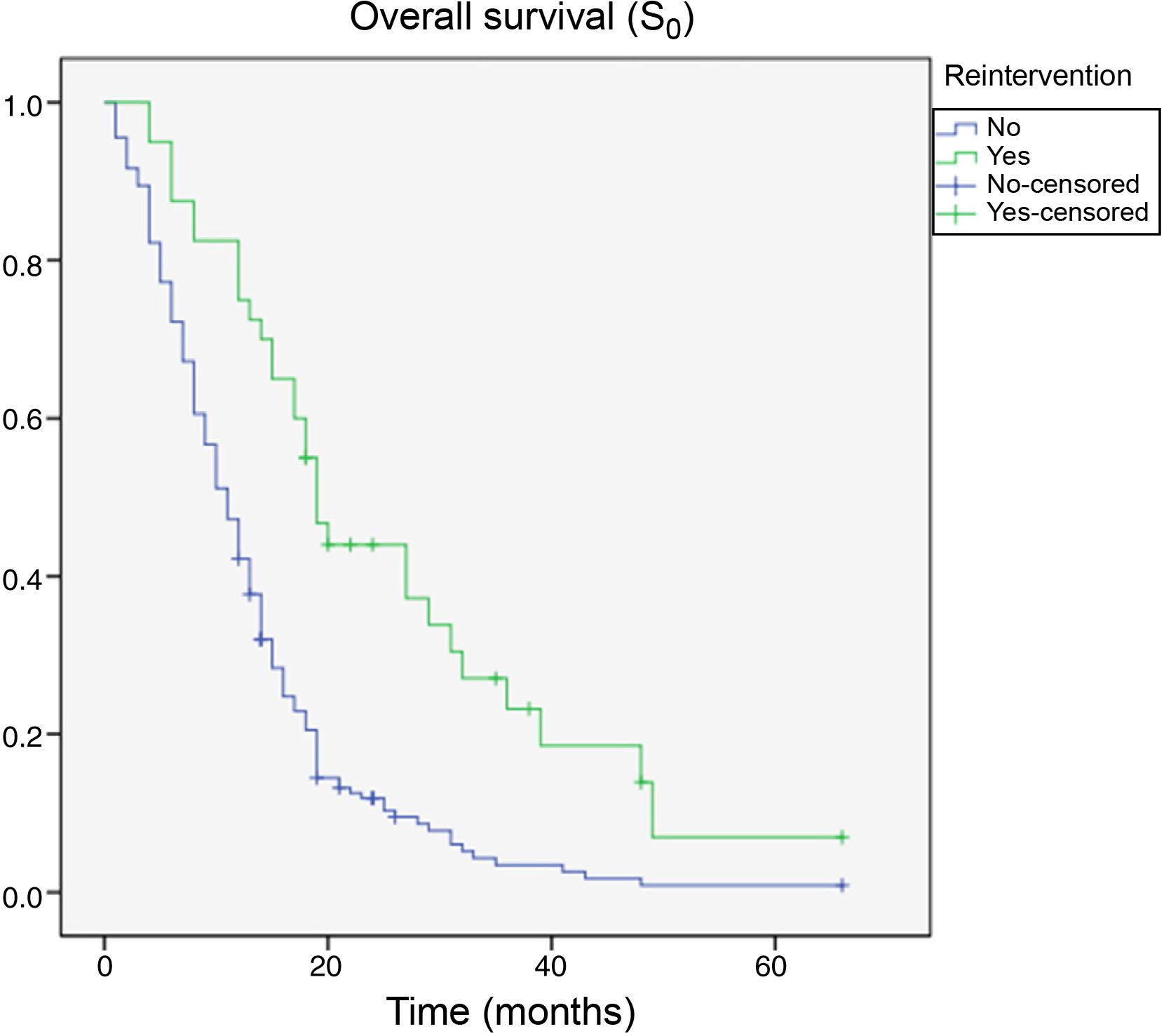
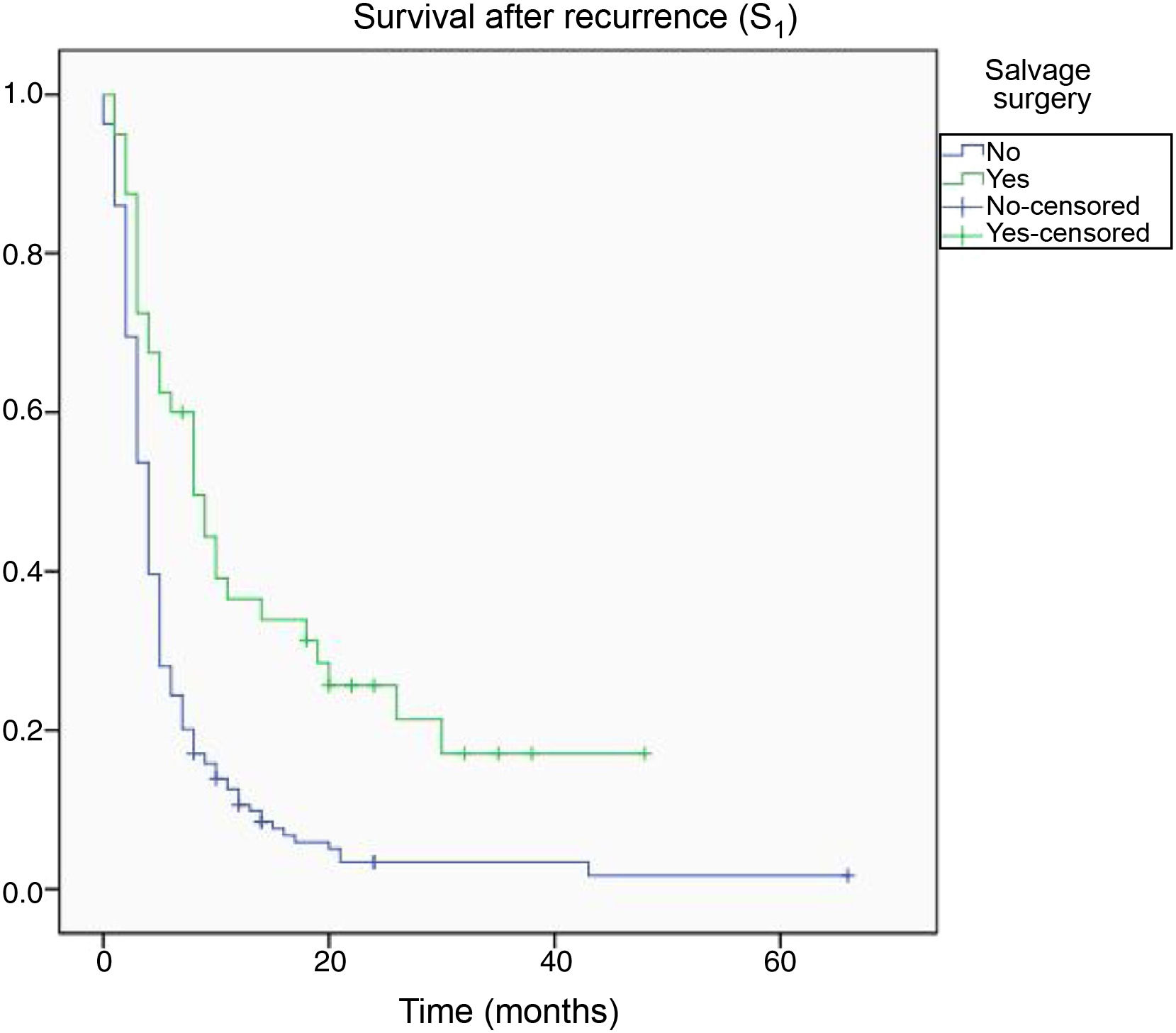
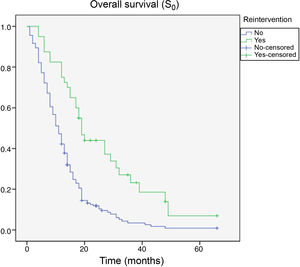
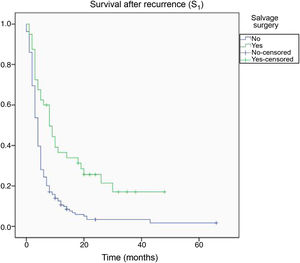
By the end of the follow-up period, 200 patients had died (89.7%), whereas 23 (10.3%) were alive. Death was directly attributed to tumour recurrence in 96 cases (48%). Median overall survival time (S0) as determined with Kaplan–Meier curves was 15.4 months (0–66.3) (Fig. 4). Median survival time after tumour recurrence was 7.8 months (0–47.4) (Fig. 5).
We observed significantly longer S0 times among patients younger than 50 years and with higher KPS scores at diagnosis (P < .05). We also observed significantly longer survival times in patients with gross-total resection in the initial surgery, with grade III tumours according to the WHO classification, and undergoing salvage surgery at the time of recurrence (Table 1). No significant differences were observed in association with sex or involvement of eloquent areas.
Association between overall survival (S0) and other variables.
| Age | ||
| < 50 years | 19.0 ± 2.7 | P < .001 |
| > 50 years | 13.0 ± 1.6 | |
| Sex | ||
| Women | 14.1 ± 2.8 | P = .107 |
| Men | 16.7 ± 2.8 | |
| KPS | ||
| 100 | 15.9 ± 1.4 | |
| 90 | 14.2 ± 1.5 | P < .001 |
| 80 | 7.2 ± 1.1 | |
| 70 | 5.1 ± 1.5 | |
| 60 | 4.8 ± 2.1 | |
| Region affected | ||
| Eloquent | 16.3 ± 2.2 | P = .337 |
| Non-eloquent | 14.8 ± 2.9 | |
| EOR | ||
| GTR | 19.3 ± 3.0 | P < .001 |
| STR | 12.6 ± 2.1 | |
| WHO tumour grade | ||
| Anaplastic astrocytoma (III) | 29.9 ± 7.2 | P < .001 |
| Glioblastoma (IV) | 12.7 ± 1.4 | |
| Salvage surgery | ||
| Yes | 21.5 ± 4.2 | P < .001 |
| No | 12.3 ± 1.7 | |
The values reported are survival time in months (mean ± standard deviation).
EOR: extent of resection; GTR: gross-total resection; KPS: Karnofsky Performance Status score; STR: subtotal resection; WHO: World Health Organization.
In the multivariate analysis, we observed significantly greater (P < .05) S0 time among patients with higher KPS score at diagnosis, gross-total resection in the initial surgical procedure, and grade III tumour, and those undergoing salvage surgery at the time of tumour recurrence (Table 2).
Multivariate Cox regression analysis of the relative risk of different variables associated with overall survival (S0).
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| KPS | 0.92 (0.89-0.95) | < .001 |
| EOR | 0.95 (0.92-0.98) | < .001 |
| WHO tumour grade | 1.67 (1.43-1.81) | < .001 |
| Reintervention | 0.94 (0.91-0.97) | < .001 |
95% CI: 95% confidence interval; EOR: extent of resection; KPS: Karnofsky Performance Status score; WHO: World Health Organization.
With respect to survival after tumour recurrence (S1), longer survival times were observed to be associated with age younger than 50 years, greater EOR1, grade III tumours, and salvage surgery (Table 3). The Cox regression model confirmed the associations between longer S1 time and grade III tumours and salvage surgery (Table 4).
Association between survival after diagnosis of tumour recurrence (S1) and other variables.
| Age | ||
| < 50 years | 13.8 ± 2.7 | P < .001 |
| > 50 years | 5.5 ± 1.7 | |
| Sex | ||
| Women | 7.6 ± 3.4 | P = .458 |
| Men | 7.9 ± 2.2 | |
| Region affected | ||
| Eloquent | 7.9 ± 2.3 | P = .234 |
| Non-eloquent | 7.1 ± 1.7 | |
| EOR | ||
| GTR | 8.9 ± 2.0 | P < .001 |
| STR | 5.6 ± 0.84 | |
| WHO tumour grade | ||
| III | 14.8 ± 4.6 | P < .001 |
| IV | 5.5 ± 1.4 | |
| Salvage surgery | ||
| Yes | 12.5 ± 2.5 | P < .001 |
| No | 4.9 ± 1.4 | |
The values reported are survival time in months (mean ± standard deviation).
EOR: extent of resection; GTR: gross-total resection; STR: subtotal resection; WHO: World Health Organization.
Multivariate Cox regression analysis of the relative risk of different variables associated with survival after tumour recurrence (S1).
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| WHO tumour grade | 1.19 (1.04–1.34) | < .001 |
| Reintervention | 0.92 (0.89–0.95) | < .001 |
95% CI: 95% confidence interval; WHO: World Health Organization.
In this series, we present our cumulative experience from the last 10 years in the treatment of high-grade glioma at a single institution, with all data gathered after the influential work published in 2005 by Stupp et al.7 That study standardised the treatment of high-grade glioma at the time of diagnosis.
During the 10-year study period, a total of 328 patients with high-grade glioma were treated at our centre, a mean of more than 30 per year; this makes our institution a high-volume centre for the treatment of this tumour.9,10
In our experience, median S0 time is 15.4 months, slightly higher than the survival time established in the current literature (around 14.6 months).7 In turn, median S1 time in our sample was 7.8 months, within the range of 6–10 months reported in the literature.11
Multivariate analysis found associations between longer S0 time and higher baseline KPS score, higher EOR, grade III tumours, and salvage surgery at the time of tumour recurrence (Table 2). The association between higher baseline KPS scores and longer survival is extensively addressed in the literature on high-grade glioma.12–15
Regarding EOR, our findings are consistent with the trend reported in recent years: more extensive, near-complete resection is associated with longer survival. This axiom, which is a constant in the great majority of solid organ malignancies, has been questioned in high-grade glioma due to the complex physiology and extreme aggressiveness of this type of tumour. In fact, some recent studies focusing on patients undergoing multiple resective procedures separated by adjuvant treatment do not report longer survival times in patients with greater EOR1; however, a benefit was observed with successive procedures achieving gross-total resection.5
Regardless of these observations, our results on EOR are consistent with those reported in the current literature. This further emphasises the importance of such tools as intraoperative neurophysiological monitoring, brain mapping, and awake surgery, which may enable more extensive resection of the tumour while ensuring safety.3 In fact, a recent study described the cost-effectiveness and cost-utility of motor mapping, due to the technique’s ability to increase the EOR in a controlled manner.16 At the other extreme, an association has been reported between disabling postoperative neurological deficits and shorter survival.17
Our experience also confirms that lower histologic grade according to the WHO criteria is associated with longer survival.18
According to our data, salvage surgery in selected patients at the time of tumour recurrence is associated with longer survival.3,5,19–21 In our series, almost one-third (32%) of patients with recurrent high-grade glioma were selected to undergo salvage surgery at the time of tumour recurrence after first-line treatment. This association between salvage surgery and increased survival has also been described in previous studies.2,3,15,20,21 This treatment is increasingly important in the light of the scarce benefits of antiangiogenic therapy with bevacizumab reported in these patients.22 However, unlike in low-grade glioma, for which the validity of repeated cytoreductive surgery is accepted,20 universal consensus is yet to be established on following the same approach for high-grade glioma.23,24 Favourable experiences have been described with such other treatments as stereotactic radiosurgery, re-irradiation,25–30 chemotherapy with maintenance of temozolomide or the introduction of nitrosoureas or other alkylating agents,31,32 low-intensity electric fields (tumour-treating fields),32 and clinical trials generally using biological therapies.31–34
A large part of the challenge in standardising the management of tumour recurrence after first-line treatment is derived from the heterogeneity of patients at the time of diagnosis.1 This heterogeneity is particularly significant in relation to the overall status of the patient, which is often characterised with the KPS score and generally influences the decision of whether or not to indicate active treatment.35–41 Patients are also heterogeneous in terms of the aggressiveness of the initial surgical procedure; the variety of anatomical localisations involved in tumour recurrence, which may affect resectability; and differences in the adjuvant therapies administered, which are generally influenced by tolerance.2 Regarding this point, and as noted by numerous authors, we must take into account the possibility of a selection bias in the patients who benefit from a therapy at the time of recurrence.2,42–45 This bias may play a role in the favourable results observed in these patients in survival analysis, leading us to overestimate the value of any treatment in this subgroup.
With a view to reducing the impact of this bias, we conducted a survival analysis from the moment of progression after first-line treatment (S1), which continued to show longer survival in patients undergoing salvage surgery.
The limitations of our study are mainly related to its retrospective design. Due to the period in which the procedures were performed, histopathological diagnosis was classified according to the 2007 WHO classification, and does not take into account the genetic characterisation published in 2016.46
Therefore, the results reported in the present study and in the literature demonstrate the importance of active, radical treatment at the time of tumour recurrence after first-line treatment, in patients meeting the criteria applied. In this sense, we believe that in the same way that clinicians have moved beyond the nihilist conception of this type of tumour at the time of initial diagnosis,1,3 we should take a similar attitude when recurrence is diagnosed. To that end, it is essential that patients are closely followed up by each and every one of the departments involved in their care, typically the neurosurgery, medical oncology, and radiotherapeutic oncology departments. This follow-up must ensure the continuity of clinical/radiological monitoring in order to promptly detect tumour recurrence, enabling timely treatment. Furthermore, it must enable the control of symptoms and medication, and particularly corticotherapy, whose maintenance is associated with poorer outcomes.47 These processes must be implemented through the creation of multidisciplinary neuro-oncology committees authorised to take binding decisions regarding the treatment of these patients.48
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank all the members of the Neuro-Oncology Committee at Hospital General Universitario Gregorio Marañón.