Stroke is the main cause of admission to neurology departments and cardioembolic stroke (CS) is one of the most common subtypes of stroke.
MethodsA multicentre prospective observational study was performed in 5 neurology departments in public hospitals in the Region of Madrid (Spain). The objective was to estimate the use of healthcare resources and costs of acute CS management. Patients with acute CS at <48h from onset were recruited. Patients’ socio-demographic, clinical, and healthcare resource use data were collected during hospitalisation and at discharge up to 30 days after admission, including data for rehabilitation treatment after discharge.
ResultsDuring an 8-month recruitment period, 128 patients were recruited: mean age, 75.3±11.25; 46.9% women; mortality rate, 4.7%. All patients met the CS diagnostic criteria established by GEENCV-SEN, based on medical history or diagnostic tests. Fifty per cent of the patients had a history of atrial fibrillation and 18.8% presented other major cardioembolic sources. Non-valvular atrial fibrillation was the most frequent cause of CS (33.6%). Data for healthcare resource use, given a mean total hospital stay of 10.3±9.3 days, are as follows: rehabilitation therapy during hospital stay (46.9%, mean 4.5 days) and after discharge (56.3%, mean 26.8 days), complications (32%), specific interventions (19.5%), and laboratory and diagnostic tests (100%). Head CT (98.4%), duplex ultrasound of supra-aortic trunks (87.5%), and electrocardiogram (85.9%) were the most frequently performed diagnostic procedures. Average total cost per patient during acute-phase management and rehabilitation was €13139. Hospital stay (45.0%) and rehabilitation at discharge (29.2%) accounted for the largest part of resources used.
ConclusionsAcute CS management in the Region of Madrid consumes large amounts of resources (€13139), mainly due to hospital stays and rehabilitation.
El ictus es la principal causa de ingreso en los servicios de Neurología, siendo el infarto cerebral cardioembólico (ICE) de los subtipos más frecuentes.
MétodosEstudio observacional, multicéntrico, prospectivo, realizado en 5 hospitales públicos de la Comunidad de Madrid, cuyo objetivo fue estimar la utilización de recursos sanitarios y costes en el manejo del ICE agudo. Se incluyeron pacientes con ICE agudo de evolución <48h. Se registraron datos sociodemográficos, clínicos y los recursos sanitarios utilizados durante el ingreso y al alta hasta 30 días desde el ingreso, incluyendo el tratamiento rehabilitador al alta.
ResultadosSe seleccionaron 128 pacientes durante 8 meses, de 75,3±11,25 años, siendo un 46,9% mujeres, con una mortalidad del 4,7%. El 100% cumplía los criterios diagnósticos del GEENCV-SEN por antecedentes o el estudio realizado. Como antecedentes clínicos, el 50% presentó fibrilación auricular, y el 18,8%, otras fuentes mayores embolígenas. La fibrilación auricular no valvular fue la causa más frecuente de ICE (33,6%). Consumo de recursos: estancia media, 10,3±9,3 días; rehabilitación durante el ingreso, 46,9%, media 4,5 días, y al alta, 56,3%, media 26,8 días; complicaciones, 32%; intervenciones hospitalarias específicas, 19,5%; pruebas diagnósticas y analíticas sanguíneas, 100%, siendo la TAC craneal (98,4%), el dúplex TSA (87,5%) y el electrocardiograma (85,9%), las diagnósticas más frecuentes. El coste total medio por paciente en la fase aguda y rehabilitación por ICE fue de 13.139€, siendo la estancia hospitalaria (45,0%) y la rehabilitación al alta (29,2%) los recursos más importantes.
ConclusionesEl manejo agudo del ICE en la Comunidad de Madrid generó un importante consumo de recursos (13.139€) debido a la asistencia hospitalaria y la rehabilitación.
Cardioembolic stroke (CS) is caused by partial or total occlusion of a cerebral or precerebral artery due to embolic material from the heart. They arise from 3 mechanisms: arrhythmias, release of material from abnormal valvular or myocardial surfaces, and paradoxical embolism.1 These infarcts are normally medium- to large-sized and tend to be located in the cortex. Symptoms often present while the patient is awake. Presentation of focal neurological signs may be immediate (in minutes) or acute (in hours) with the highest level of neurological impairment at disease onset. Both presence of an emboligenic heart disease and absence of significant arterial occlusion or stenosis are necessary conditions for a diagnosis of CS.2
CS accounts for 14% to 30% of all cerebral infarcts.3 According to a recent observational study conducted in Spain including more than 6000 stroke patients, 87.6% of all strokes were cerebral infarcts, 26.2% of which were cardioembolic strokes.4–6 This type of cerebral infarct has a crude incidence rate of 28 cases per 100000 inhabitants per year and the crude prevalence rate among the elderly is estimated at 8 cases per 1000 inhabitants.7
Cerebrovascular diseases are the leading cause of death in women and the second most common in men in Spain.8 Stroke is the fourth greatest cause of years of life lost due to disability, the leading cause of long-term disability in adults, and the second greatest cause of dementia.9 More than 18% of premature deaths are caused by stroke, which is responsible for a loss of 12 potential years of life per 10000 inhabitants, with greater losses among men.9,10 Cardioembolic strokes are larger infarcts that may affect multiple territories and present greater recurrence rates. Likewise, patients with CS have a poorer prognosis than those with atherothrombotic stroke, with 10-year survival rates of less than 45%.11
Stroke is the most frequent cause of admission and long hospital stays in neurology departments, and the tools required to diagnose it are costly.12 Healthcare costs generated in the first year after diagnosis are considerable due to hospital stays and rehabilitation; in fact, hospital stays represent up to 50% of the total costs accrued during this period. Rehabilitation and transport after discharge generate the highest costs associated with outpatient care.13,14
New oral anticoagulants provide new therapeutic possibilities for CS prevention, but they also incur greater direct costs than the available alternatives.
In view of the high incidence of stroke (especially among the elderly), population ageing, and above all the high costs of new therapies and the severity of CS (resulting in greater use of healthcare resources), it is essential to understand the cost of managing patients with CS to rationalise and appropriately allocate monetary resources. This knowledge may serve as a base for future studies on costs or prevention strategies.
The main purpose of the CODICE study is to determine the healthcare resources and direct costs associated with managing patients with acute CS hospitalised in public healthcare centres in the Region of Madrid, from the perspective of the Spanish Health System.
Patients and methodsThe CODICE study is a multicentre prospective observational study based on normal clinical practice. It was conducted in 5 neurology departments with or without a stroke unit in healthcare centres pertaining to the public healthcare network in the Region of Madrid: Hospital Universitario de La Princesa, Hospital General Universitario Gregorio Marañón, Hospital Universitario Príncipe de Asturias, Hospital Universitario Fundación Alcorcón, and Hospital Universitario Infanta Sofía.
The study was approved by the General Directorate for Ordinance and Inspection of the Madrid regional health authority, and by the Clinical Research Ethics Committee at Hospital Universitario de La Princesa, which acted as the centre of reference. Likewise, the study was classified by the Spanish Agency for Medicines and Medical Devices. Informed consent forms were signed by all patients included in the CODICE study, or by their authorised carers.
Study populationThe study included patients aged ≥18 years and diagnosed with CS as defined by the Spanish Society of Neurology.2 Patients had shown evidence, in the absence of other aetiologies, of such emboligenic heart diseases as intracardiac thrombi or tumours, rheumatic mitral stenosis, mitral or aortic valve replacement, endocarditis, atrial fibrillation, sick sinus syndrome, left ventricular aneurysm or akinesia secondary to acute myocardial infarction, recent acute myocardial infarction (<3 months), or global cardiac hypokinesia or dyskinesia. All patients had been admitted to the neurology department within 48hours of onset. We excluded all patients with a diagnosis of stroke types other than CS or transient ischaemic attack, as well as patients experiencing a stroke episode during a hospital stay due to unusual cardioembolic causes such as embolisms secondary to cardiac procedures.
We selected patients consecutively in a competitive recruitment process spanning an 8-month period (December 2011-July 2012). We calculated the sample size (140 patients) using GRANMO version 7.10 based on the estimated number of patients with a new episode of stroke in the Region of Madrid (1625 patients), the crude incidence rate of stroke, and the proportion of patients with cardioembolic stroke,1,15 with an alpha risk value of 0.05, a precision of ±0.165 units using a 2-tailed comparison, and assuming a drop-out rate of 7%.
Design and study variablesStudy data were collected prospectively using case report forms which were completed by the researcher. The study was structured in 2 phases: the initial consultation, coinciding with the patient's admission visit, and follow-up after discharge, conducted 30 days after the date of admission via a telephone call to the patient and/or carer.
During the initial consultation, we gathered sociodemographic and clinical data, as well as data related to resource use during the hospital stays, using computer databases, records, medical histories, and discharge reports. We also collected information about prescribed chronic pharmacological treatment and estimated rehabilitation needs after discharge, including type of rehabilitation and expected duration. The level of physical disability after CS was measured on the modified Rankin Scale (mRS), according to which higher scores correspond to higher levels of disability: the maximum score (6) indicates death.16
In the follow-up telephone calls, we collected data on resources used after discharge and the patients’ clinical situation (death, complications, readmissions, visits to the emergency department), and on rehabilitation treatment after discharge.
Statistical analysisThe data obtained were entered in a database and analysed with PASW Statistics® v 18.0. We performed a descriptive analysis of the clinical variables and those related to the disease and its management. The mean, standard deviation (SD), 95% confidence intervals, variance, standard error, 5% trimmed mean, median, and minimum and maximum values were calculated for quantitative variables, and frequency distributions and percentages for qualitative variables. We also analysed the cost of resource consumption per patient.
Cost estimationsIn the CODICE study, direct costs of acute CS management in the Region of Madrid include the costs of resource use during hospital stays, the cost of rehabilitation treatment after discharge, and the cost of resources consumed between discharge and 30 days after admission (readmissions, visits to the emergency department, long-term pharmacological treatment).
The estimated cost per patient was calculated based on consumption of health resources per patient and the unit costs of each resource.
Unit costs of healthcare resources were calculated based on market prices obtained from the e-Salud healthcare costs database (Oblikue Consulting).17 The cost of pharmacological treatments (retail price−VAT) was calculated using the prices published in the database kept by Spain's General Council of Official Pharmacy Associations.18 Costs are presented in euros (€) in 2012 prices.
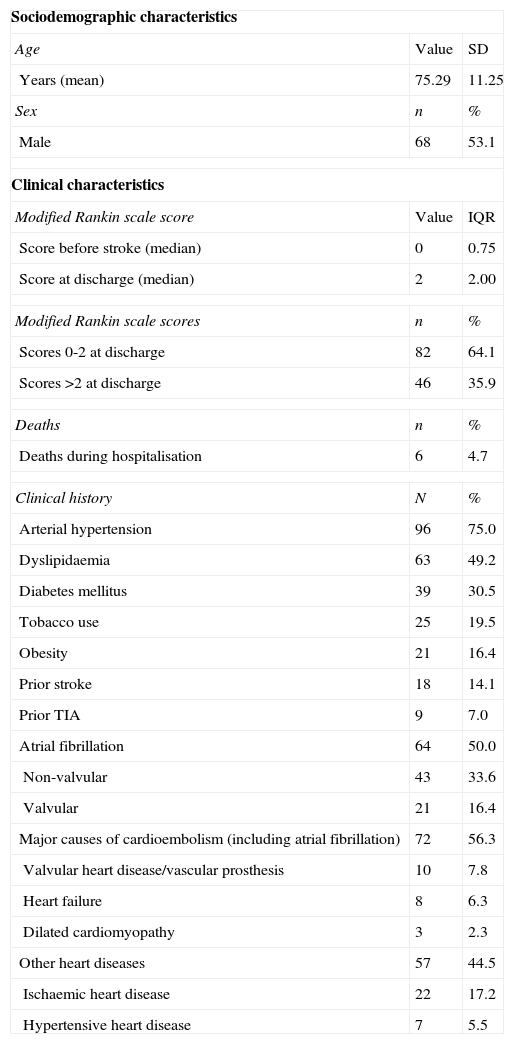
ResultsBaseline patient characteristicsThe study included 128 patients; their clinical and sociodemographic characteristics are listed in Table 1.
Patients’ sociodemographic and clinical characteristics (n=128).
| Sociodemographic characteristics | ||
| Age | Value | SD |
| Years (mean) | 75.29 | 11.25 |
| Sex | n | % |
| Male | 68 | 53.1 |
| Clinical characteristics | ||
| Modified Rankin scale score | Value | IQR |
| Score before stroke (median) | 0 | 0.75 |
| Score at discharge (median) | 2 | 2.00 |
| Modified Rankin scale scores | n | % |
| Scores 0-2 at discharge | 82 | 64.1 |
| Scores >2 at discharge | 46 | 35.9 |
| Deaths | n | % |
| Deaths during hospitalisation | 6 | 4.7 |
| Clinical history | N | % |
| Arterial hypertension | 96 | 75.0 |
| Dyslipidaemia | 63 | 49.2 |
| Diabetes mellitus | 39 | 30.5 |
| Tobacco use | 25 | 19.5 |
| Obesity | 21 | 16.4 |
| Prior stroke | 18 | 14.1 |
| Prior TIA | 9 | 7.0 |
| Atrial fibrillation | 64 | 50.0 |
| Non-valvular | 43 | 33.6 |
| Valvular | 21 | 16.4 |
| Major causes of cardioembolism (including atrial fibrillation) | 72 | 56.3 |
| Valvular heart disease/vascular prosthesis | 10 | 7.8 |
| Heart failure | 8 | 6.3 |
| Dilated cardiomyopathy | 3 | 2.3 |
| Other heart diseases | 57 | 44.5 |
| Ischaemic heart disease | 22 | 17.2 |
| Hypertensive heart disease | 7 | 5.5 |
TIA: transient ischaemic attack; SD: standard deviation; IQR: interquartile range.
Mean±SD age was 75.29±11.25 years (range, 30-95 years). Men accounted for 53.1% of the patients. The hospital mortality rate was 4.7%. The median mRS score at discharge was 2 (interquartile range, 2.0), which indicates that the patients display mild disability but are functionally independent. The median mRS score prior to stroke was 0 (interquartile range, 0.75). Scores at discharge were a mean of 1.57 points higher than baseline scores; 35.9% of patients scored higher than 2 on the date of discharge.
All patients met the diagnostic criteria for CS established by the Spanish Society of Neurology's Cerebrovascular Disease Study Group, based on either their medical histories or results from the tests performed at admission. Potential causes of embolism were the most frequent vascular risk factors (present in 57%); atrial fibrillation was detected in 50% of the patients and other major cardioembolic sources were found in 18.8%. Arterial hypertension and dyslipidaemia were also found in a high percentage of patients. According to clinical assessments at discharge, all patients presented at least one cause of CS, the most frequent being non-valvular atrial fibrillation (present in 33.6%, or 50% counting all types of atrial fibrillation). Other causes of CS were dilated cardiomyopathy (LVEF<30%), recent congestive heart failure (5.5%), or valvular disease (4.7%).
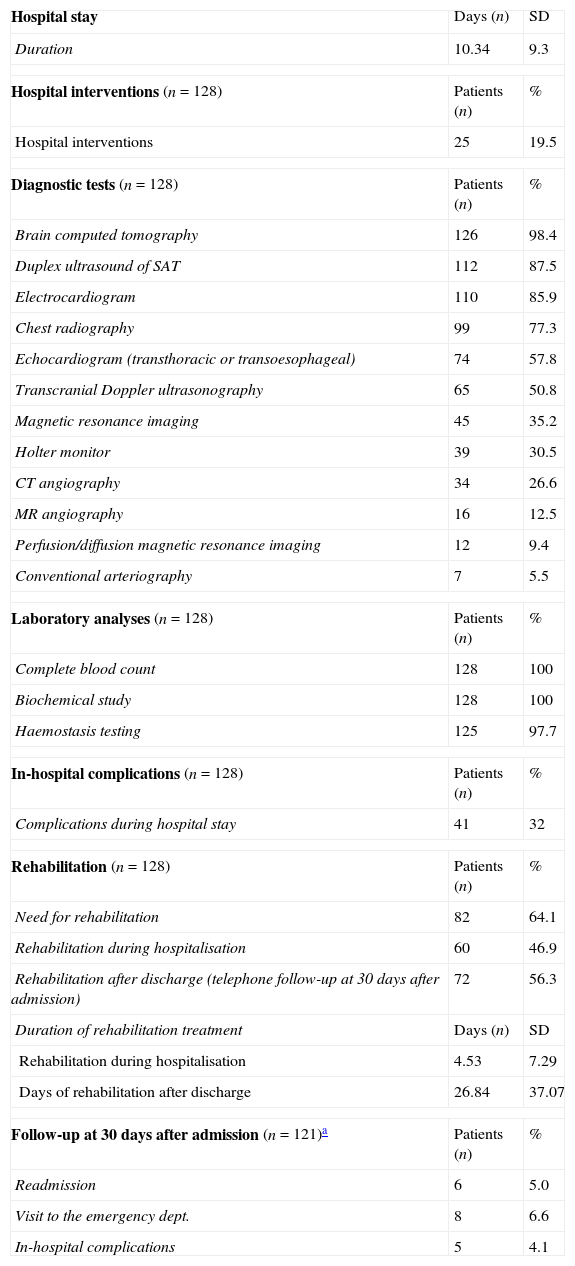
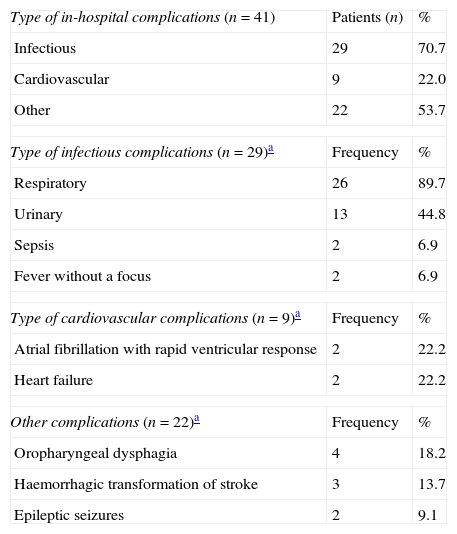
Use of healthcare resourcesResource consumption by patients included in the study is summarised in Table 2. Hospital stays lasted a mean of 10.34±9.30 days. During hospital stays, 32% of the patients presented complications of some type; the most frequent complications were attributed to infection (Table 3).
Healthcare resource utilisation (n=128).
| Hospital stay | Days (n) | SD |
| Duration | 10.34 | 9.3 |
| Hospital interventions (n=128) | Patients (n) | % |
| Hospital interventions | 25 | 19.5 |
| Diagnostic tests (n=128) | Patients (n) | % |
| Brain computed tomography | 126 | 98.4 |
| Duplex ultrasound of SAT | 112 | 87.5 |
| Electrocardiogram | 110 | 85.9 |
| Chest radiography | 99 | 77.3 |
| Echocardiogram (transthoracic or transoesophageal) | 74 | 57.8 |
| Transcranial Doppler ultrasonography | 65 | 50.8 |
| Magnetic resonance imaging | 45 | 35.2 |
| Holter monitor | 39 | 30.5 |
| CT angiography | 34 | 26.6 |
| MR angiography | 16 | 12.5 |
| Perfusion/diffusion magnetic resonance imaging | 12 | 9.4 |
| Conventional arteriography | 7 | 5.5 |
| Laboratory analyses (n=128) | Patients (n) | % |
| Complete blood count | 128 | 100 |
| Biochemical study | 128 | 100 |
| Haemostasis testing | 125 | 97.7 |
| In-hospital complications (n=128) | Patients (n) | % |
| Complications during hospital stay | 41 | 32 |
| Rehabilitation (n=128) | Patients (n) | % |
| Need for rehabilitation | 82 | 64.1 |
| Rehabilitation during hospitalisation | 60 | 46.9 |
| Rehabilitation after discharge (telephone follow-up at 30 days after admission) | 72 | 56.3 |
| Duration of rehabilitation treatment | Days (n) | SD |
| Rehabilitation during hospitalisation | 4.53 | 7.29 |
| Days of rehabilitation after discharge | 26.84 | 37.07 |
| Follow-up at 30 days after admission (n=121)a | Patients (n) | % |
| Readmission | 6 | 5.0 |
| Visit to the emergency dept. | 8 | 6.6 |
| In-hospital complications | 5 | 4.1 |
SD: standard deviation; MR: magnetic resonance; CT: computed tomography; SAT: supra-aortic trunks.
In-hospital complications (n=41).
| Type of in-hospital complications (n=41) | Patients (n) | % |
| Infectious | 29 | 70.7 |
| Cardiovascular | 9 | 22.0 |
| Other | 22 | 53.7 |
| Type of infectious complications (n=29)a | Frequency | % |
| Respiratory | 26 | 89.7 |
| Urinary | 13 | 44.8 |
| Sepsis | 2 | 6.9 |
| Fever without a focus | 2 | 6.9 |
| Type of cardiovascular complications (n=9)a | Frequency | % |
| Atrial fibrillation with rapid ventricular response | 2 | 22.2 |
| Heart failure | 2 | 22.2 |
| Other complications (n=22)a | Frequency | % |
| Oropharyngeal dysphagia | 4 | 18.2 |
| Haemorrhagic transformation of stroke | 3 | 13.7 |
| Epileptic seizures | 2 | 9.1 |
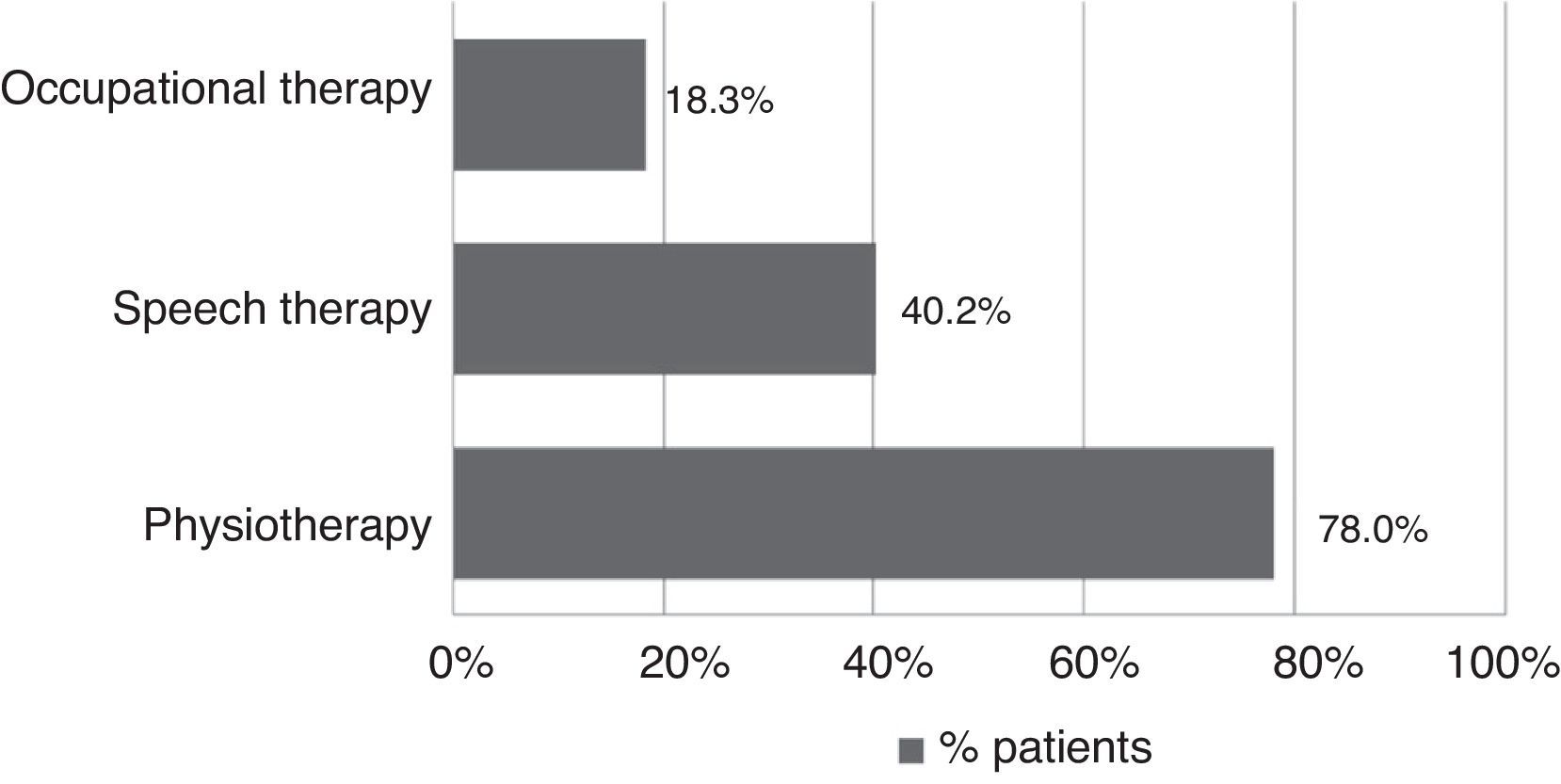
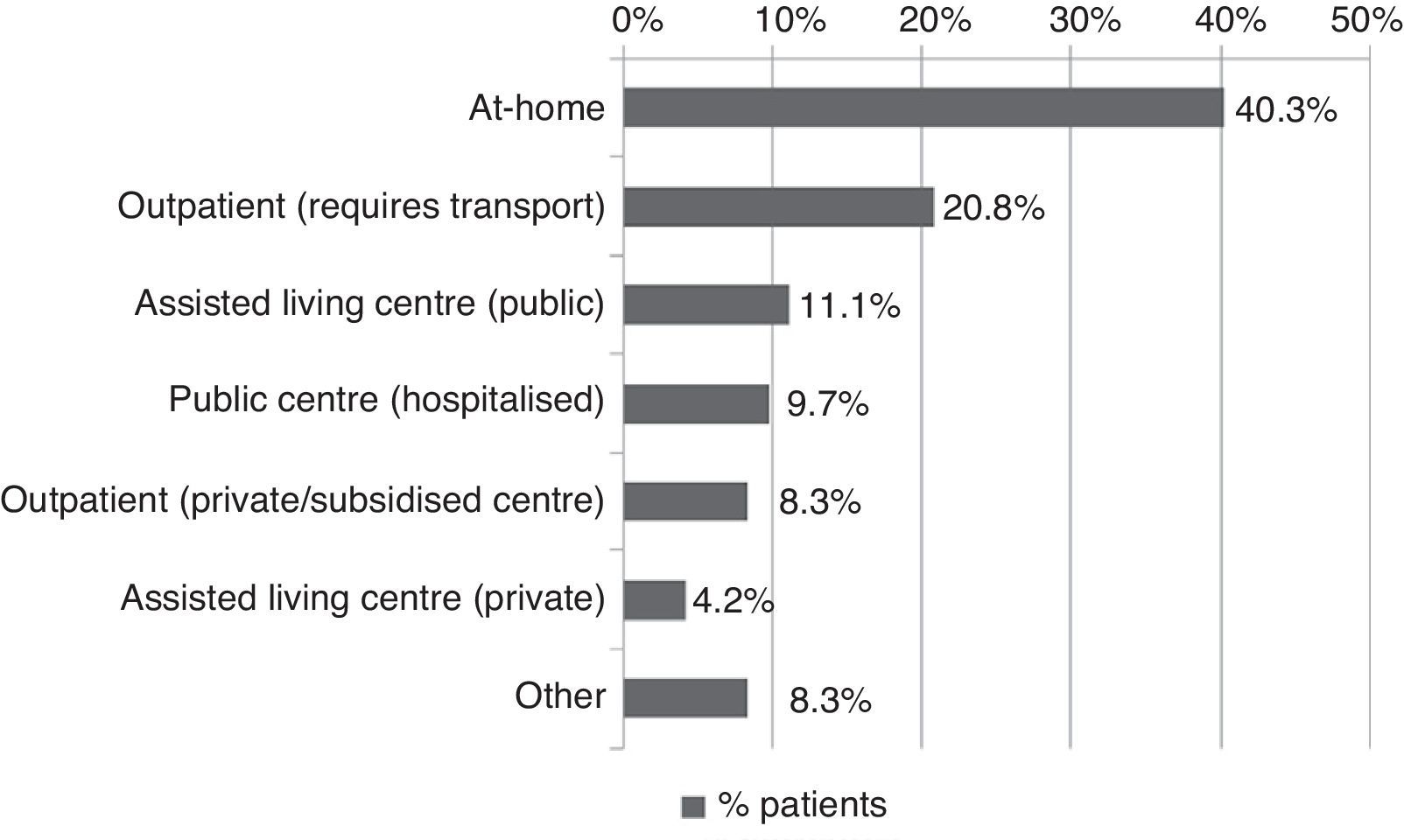
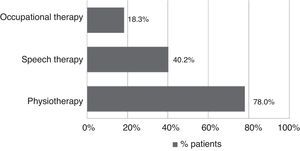
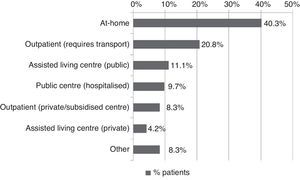
All patients received pharmacological treatment during their hospital stay and after discharge, and all of them underwent some type of analysis or diagnostic test. In order to confirm diagnosis of CS, all patients underwent brain imaging studies, as well as studies of the carotid artery that yielded negative results. Likewise, they all had a history of cardioembolic risk factors or showed cardioembolic findings in electrocardiogram or echocardiogram tests. The most frequent diagnostic tests were brain computed tomography (98.4%), duplex ultrasound of the supra-aortic trunks (87.5%), electrocardiogram (85.9%), and chest radiography (77.3%). The most frequent analyses were complete blood counts and biochemical studies, which were conducted in all patients, as well as haemostasis tests (97.7%). Rehabilitation was prescribed to 82 patients (64.1%); physiotherapy and speech therapy were the most frequently prescribed rehabilitation treatments. During their hospital stays, 46.9% of patients underwent rehabilitation; after time of discharge, that percentage rose to 56.3%. Mean duration of rehabilitation was 31.37±40.01 days per patient. Broken down by treatment location, after discharge most patients underwent rehabilitation at home (40.3%), while 20.8% underwent rehabilitation on an outpatient basis (Figs. 1 and 2).
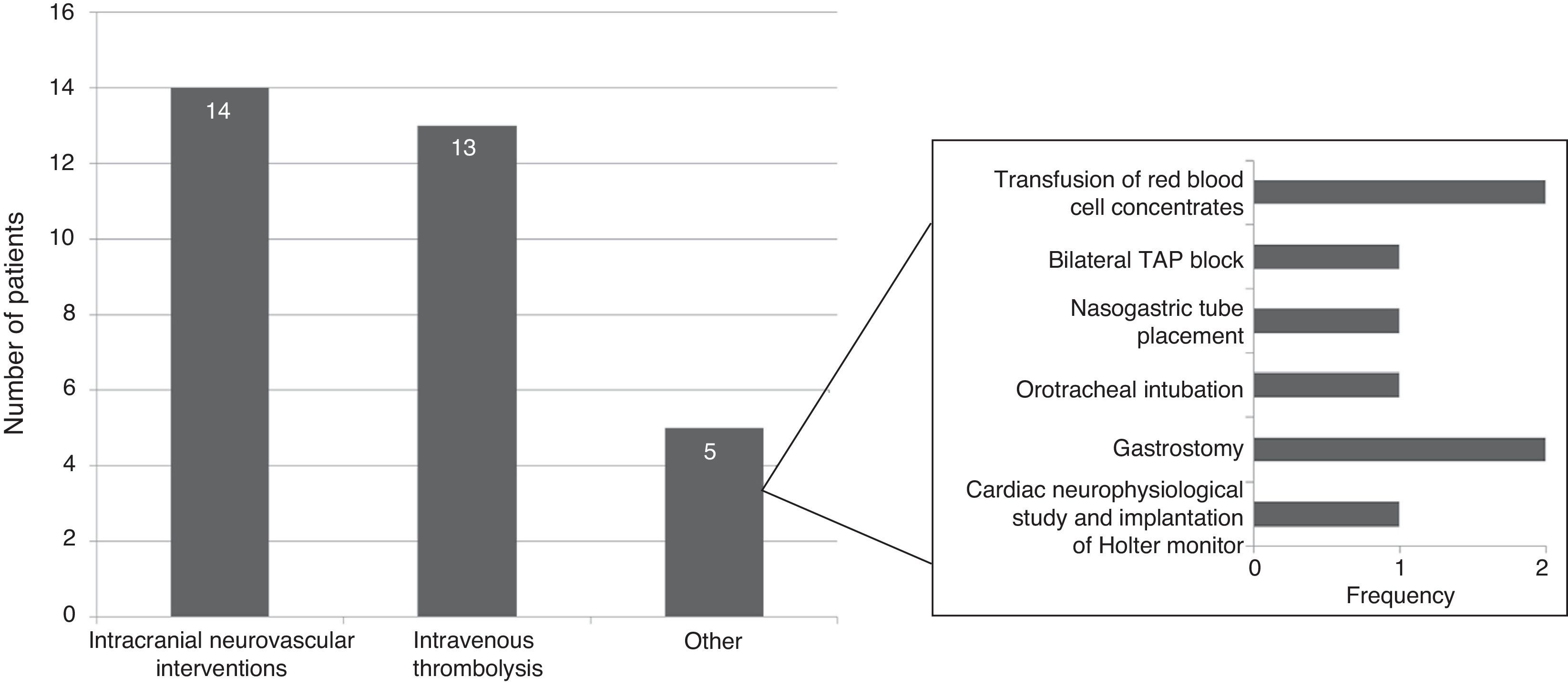
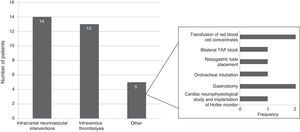
Specific in-hospital interventions were required by 19.5% of the patients; the most frequent were intracranial neurovascular interventions (56%) and intravenous thrombolysis (52%) (Fig. 3).
Follow-up at 30 days after admissionWe conducted telephone interviews with 121 patients (94.5%) after discharge and at 30 days after admission. While 6.6% of patients remained in hospital, 11.6% had been institutionalised in residential centres, and 5% had been readmitted to hospital, due to stroke complications in one third of these cases. During this period, 6.6% of patients visited the emergency department and 4.1% experienced complications.
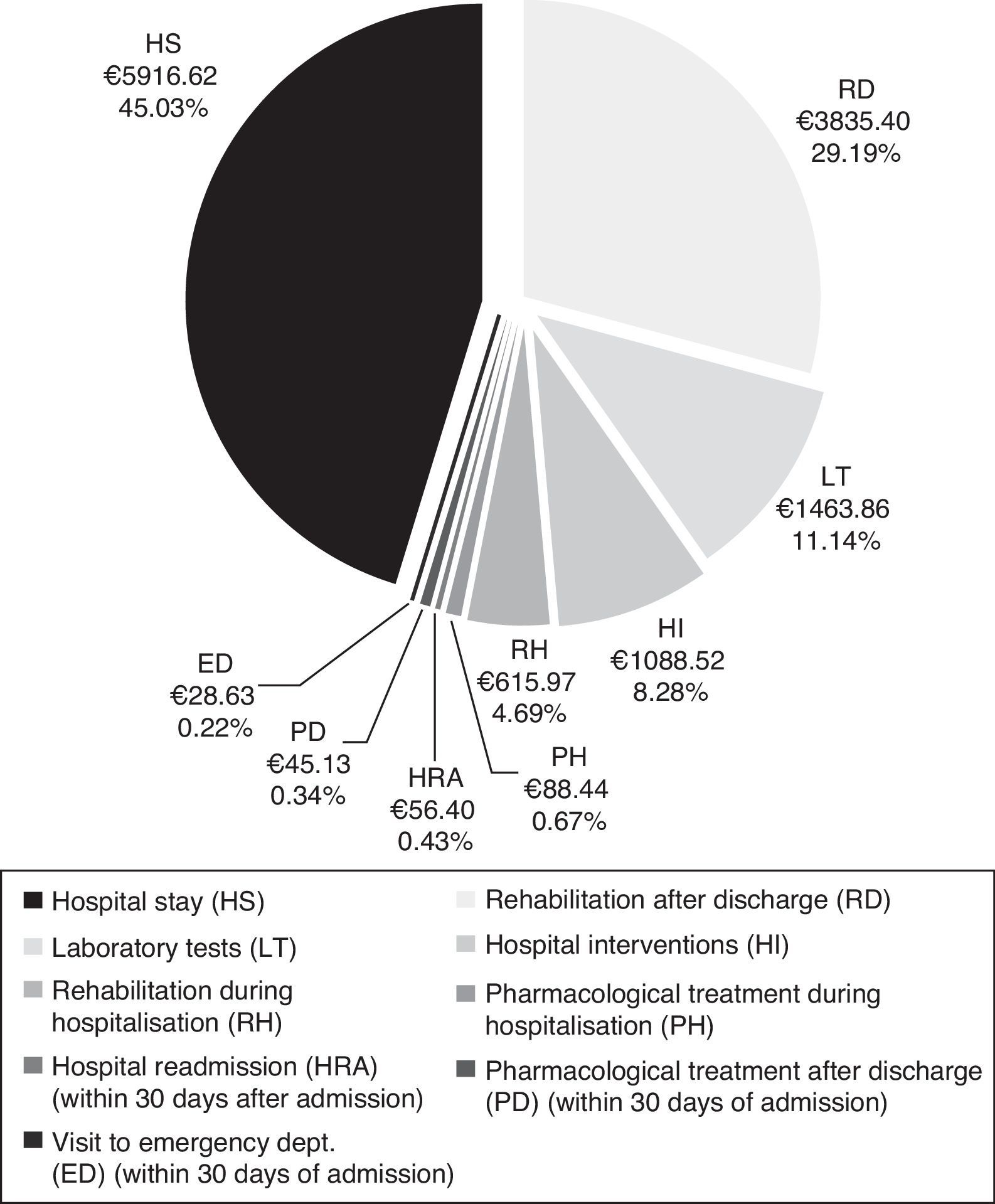
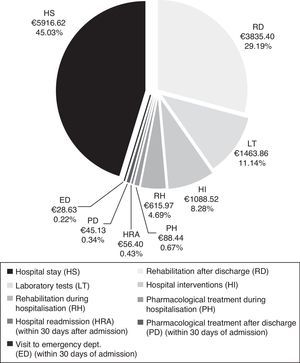
CostsMean total cost of acute CS management in the Region of Madrid was €13138.97 per patient. Hospital stays (€5916.62) and rehabilitation after discharge (€3835.40) accounted for the largest part of the total cost (Fig. 4). The mean cost per patient during the hospital stay was €9173.42.
DiscussionCS management requires greater investments during the initial period after the episode; the costs during the first year are particularly high compared to subsequent years.13,19 This being the case, it is essential that we measure the costs generated during the first few months after the episode, especially in the acute phase during hospitalisation and functional recovery (rehabilitation), since these 2 stages generate the highest costs. The total financial burden of CS management also includes other types of costs, such as social costs and those related to long-term healthcare and rehabilitation, which are not included among the objectives of the present study.
Total cost of CS management in the Region of Madrid was calculated at €13139 per patient, of which roughly 70% corresponds to patient management during the hospital stay. The cost of hospitalisation (€5917) accounted for the largest part of the hospital resources consumed. In addition, patients with CS may need specific interventions that increase resource use during hospitalisation, as did 19.5% of the patients in our study. The most frequent interventions were intracranial neurovascular surgery and intravenous thrombolysis, which were performed on more than half of the patients undergoing hospital interventions. Nearly one third of all patients (32%) experienced hospital complications which were infectious in most cases. At €3835, rehabilitation therapy, primarily employed after discharge, was another important item in the costs associated with CS management.
The participating centres belong to the Madrid Healthcare Service, which launched a stroke care plan in 2008 providing a comprehensive approach to stroke management and establishing specific action protocols and efficient allocation of resources.20 The CODICE study provides relevant data about current specific characteristics of CS management and resource use after implementation of the stroke care plan in the Region of Madrid.
Several studies conducted in Spain address stroke management costs13,19,21–27; however, they provide no specific data regarding direct costs of CS management, and their results are therefore difficult to compare to those from the CODICE study.
One study of the socioeconomic costs of stroke in Spain compared stroke-related costs in patients with and without non-valvular atrial fibrillation.26 This study differs from our own in 2 ways: it was conducted only in neurology departments with stroke units, and researchers estimated costs from a social perspective. Preliminary data from this study reported a hospital cost of €6428 associated with each stroke episode.21 Another study conducted in Spain estimated stroke management costs and quality of life of stroke survivors during the first 3 years after the episode and reported a direct cost of €5007 corresponding to the first year (2004).19 Unlike in the CODICE study, data presented by López-Bastida et al. were obtained from mean costs per diagnosis-related group in 18 Spanish healthcare centres. In another study, the mean cost of the diagnosis-related group (2008) for stroke with infarct at Hospital General Universitario de Valencia was €4227.15.23 Fernández de Bobadilla et al. estimated the mean crude annual stroke-related cost per patient (2006) in normal clinical practice in Spain at €2590.36, including primary care direct costs (€1630.06).25 Mean direct costs of hospital care for the first year after stroke were €2990.70 in 2004, plus €80.50 and €466.10 for rehabilitation and transportation, respectively. These costs were calculated from a social perspective in a retrospective study covering a 3-year period.13 A cost study in the Basque Country estimated the mean transitional care cost for cerebral infarct at €2587 (2002) for hospitalisation in the acute phase, and €256.80 for a readmission. Costs derived from inpatient and outpatient rehabilitation treatment were estimated at €925.40 and €488.70, respectively.22 The results from the CODICE study cannot be directly compared to those from the studies mentioned above since they applied different methodologies and inclusion criteria and were conducted in different years.
Data quality depends largely on the study design. Prospective observational studies provide optimal scientific evidence for this type of data, since information is more precise when it is gathered during patient management; this approach prevents the data loss inherent to retrospective studies.28 Furthermore, these studies offer a higher-quality design since they have to be classified by the Spanish Agency for Medicines and Medical Devices and approved by an authorised Clinical Research Ethics Committee.29 Another advantage of prospective longitudinal studies is that they enable data collection during normal clinical practice, which provides real data corresponding to patient management at each hospital, which will depend on the severity and therapeutic needs in each case.
However, the present study does have some limitations. Resource consumption in the early days after discharge was estimated based on telephone interviews with patients or carers, and some resources may not have been mentioned, despite the short time period in question. The duration of rehabilitation treatment after discharge was calculated based on the amount of time prescribed by the rehabilitation doctor while patients were hospitalised; these data were confirmed by telephone interview at 30 days after admission.
We were unable to gather NIHSS-based neurological assessment data from all centres since some of them lack an on-call neurologist and therefore did not calculate NIHSS scores at admission. According to the available data, the level of severity at discharge is thought to be lower than usually reported; this may be explained by the high percentage of patients who receive reperfusion therapy during the acute phase.
All 5 public hospitals included in our study are located in the Region of Madrid, so our results may not be representative of other Spanish regions due to local differences in resource use.30
This analysis does not compare costs between neurology departments with and without stroke units, but it would be interesting to include this variable in future analyses of the CODICE study and other research projects.
The intensive health resource consumption involved in acute CS management, which incurs a mean total cost of €13139 per patient, owes largely to hospital stays. This is partly due to the high level of specialisation, diagnostic techniques, and specific therapeutic interventions these patients need. In addition to CS management during the acute phase, rehabilitation was needed to treat the sequelae, which also contributed to the monetary resources consumed by the Madrid Healthcare Service.
FundingThis study was funded by Pfizer S. L. U.and Bristol-Myers Squibb S. A.
Conflicts of interestJosé Vivancos Mora and Antonio Gil Núñez have received fees from Pfizer S. L. U. for their role as research consultants and coordinators. All researchers have received fees from Pfizer S. L. U. for participating in the study. Miguel Ángel Casado Gómez, Covadonga Torres González, and Fernando de Andrés Nogales, who were responsible for study monitoring and data analysis, are employed by PORIB. Marina de Salas Cansado and Javier Soto Álvarez are employed by Pfizer S. L. U.
Francisco Javier Barriga Hernández, Laura Izquierdo Esteban, Fernando Díaz Otero, José Vivancos Mora, M. Ángeles Ortega Casarrubios, Laura Castillo Moreno, Teresa Montojo Villasanta, Mónica Álvarez Moreno, Pablo Bandres Hernández, Carlos Ignacio Gómez-Escalonilla Escobar, Inmaculada Puertas Muñoz, Alicia Parra Santiago, M. Paz Martín Torres, Álvaro Ximénez-Carrillo, and Gemma Reig Roselló.
The names of the members of the CODICE study research group are listed in Annex.
Please cite this article as: de Andrés-Nogales F, Vivancos Mora J, Barriga Hernández FJ, Díaz Otero F, Izquierdo Esteban L, Ortega-Casarrubios MÁ, et al. Utilización de recursos sanitarios y costes asociados al manejo de los pacientes con infarto cerebral cardioembólico agudo en la Comunidad de Madrid: Estudio CODICE. Neurología. 2015;30:536–544.
Preliminary data from this study were presented at the 15th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) under the title ‘Health care resource utilisation and costs of cardioembolic stroke in the region of Madrid, Spain: preliminary results of CODICE study’ (Berlin, 3-7 November 2012). They were also presented at the XXXIII Health Economics Conference (Santander, 18-21 June 2013) of the Spanish Health Economics Association under the title ‘Use of healthcare resources and costs of acute cardioembolic stroke management in hospitalised patients in the Region of Madrid: the CODICE study’.