As life expectancy increases, and the prevalence of cognitive impairment and dementia continues to grow, the number of patients with cognitive complaints seen in Primary Care or specialized out-patient clinics has increased in the last few years. The assessment of these patients requires time and a step-by-step organization to optimize medical resources.
DevelopmentThis review presents the most important dementia screening tools with Spanish validation. We focus on those that are brief (less than 10min) and easy to use in Primary Care settings. Two groups of tests can be distinguished: brief cognitive tests and functional activities scales. The first can be considered as a part of the mental status examination, and the second as an organized history taking. Informant questionnaires and the possibility of self-administered cognitive tests are briefly reviewed.
ConclusionThere are no ideal screening tests. The election of the most appropriate will depend on the physician's time and knowledge of each test. It is advisable to be familiar with a reduced number of tests, and be aware of their strengths and limitations. Finally, we suggest personal recommendations for the most useful tests in each clinical setting.
Con el envejecimiento de la población y el consiguiente aumento de la prevalencia de deterioro cognitivo y demencia, el número de pacientes que se presentan con quejas cognitivas en las consultas de atención primaria y especializada se ha disparado en los últimos años. Su evaluación requiere tiempo y una organización asistencial escalonada para optimizar recursos.
DesarrolloEn la presente revisión se presentan los principales test de cribado de demencia que han sido estudiados y validados en nuestro medio, destacando aquellos más breves (tiempo de administración menor de 10 minutos) y adecuados para atención primaria. Se pueden dividir en dos grandes grupos: test cognivos breves (conceptualemente una parte de la exploración neurológica) y escalas funcionales (una parte de la anamnesis, recogida de forma estructurada). Se revisa también la utilidad de los test del informador y la posibilidad de utilizar test autoadministrados.
ConclusiónPuesto que no existe el test ideal, la utilización de un test u otro dependerá de la disponibilidad de tiempo y la experiencia del administrador. Conviene familiarizarse con un número reducido de ellos, y conocer tanto sus ventajas como sus limitaciones. Se proponen unas recomendaciónes personales sobre los test más adecuados en cada nivel asistencial.
Population ageing has brought with it an increase in the prevalence of dementia. It is estimated that close to 24 million people around the world suffer some form of dementia and its prevalence will double every 20 years.1 Early detection and diagnosis have become an important public health problem that has yet to be resolved as various international studies have shown.2,3 These studies reflect a mean delay of between 8 and 32 months between the onset of symptoms and diagnosis, as well as significant dissatisfaction of caregivers with the ability of Primary Care physicians to diagnose the condition in its initial stages. The benefits of an early diagnosis include doing away with uncertainty, confirming suspicions, enhancing the understanding of the problem, promoting strategies for coping with the disease, facilitating personal planning, having access to treatment, and providing access to social support measures.4 To date, not a single country has proposed systematic detection in the population of patients with dementia in Primary Care, perhaps due to the limited efficacy of the treatments available, in spite of the fact that it is well known that the percentage of undiagnosed cases is extremely high.5 In general, “case finding” is recommended6; this consists of active screening carried out by the Primary Care physician when facing the detection of a risk factor for the disease. In the case of dementia, these risk factors would be complaints of memory loss, cognitive impairment, or functional decline.
The diagnosis of dementia is clinical, with a limited role so far for biomarkers, and requires a considerable amount of time to collect the information needed for patient anamnesis and examination. Moreover, in most cases, the onset of dementia is gradual and may be difficult to distinguish from ailments such as normal ageing, depression, or pre-existing low intelligence. This time demand placed on doctors collides with the reality of almost all Primary Care clinics and with many neurology outpatient clinics. The mean time per consultation in Primary Care in Europe is 10.7min,7 and in Spain it is approximately 5min, with frequent requests that it be extended to at least 10min.8
The need to elaborate simple, fast tests enabling physicians to detect those individuals who may suffer from dementia or cases in which the diagnosis is doubtful and who require referral for specialized evaluation in just a few minutes with an objective measure is born out of this lack of physician time. The task of providing a measure of a person's cognitive abilities in just a few minutes is a formidable task, which may explain perhaps why there have been such an enormous number of tests proposed and studied in recent years. In this review article, which in no way seeks to be systematic, the authors intend to provide general practitioners with a practical view of the short tests and scales for the detection of dementia that are most widely used in our setting.
What is a short test?The tests that will be reviewed in this article are designed to detect and screen for dementia, and are not for diagnostic purposes, which will always be clinical. It is wise to avoid phrases such as, “the patient has dementia because he/she scores below the cut-off on the test…”. As we have previously commented, the diagnosis of dementia is made on a clinical basis; no test can substitute the clinician when establishing the diagnosis.
The ideal short test should meet the following requirements: (a) it must be quick and easy to administer in order to be accepted by professionals; (b) it must be well tolerated and accepted by patients; (c) it must be easy to score, and (d) in must be independent of language, culture, or level of education. Furthermore, it must meet a series of methodological requirements, for instance: (a) good internal consistency; (b) high inter-rater reliability; (c) have good concurrent validity; (d) it must have good predictive and criterion validity, and (e) it must have comparative norms. The interested reader has outstanding reviews of the methodology of diagnostic tests, both general tests,9 as well as those specifically for dementia.10,11 In this regard, so as to avoid the heterogeneity of the methods used in validating diagnostic tests, initiatives recently started up, for example the STARD12 (Standards for the Reporting of Diagnostic Accuracy) are of great importance. They attempt to improve the quality of the articles published, verifying that they satisfy a series of requirements, and have already been adopted by many journals, for example, Neurology.
The dementia screening tests can be divided into 4 different kinds: cognitive tests, self-administered tests, informant-rated tests, and functional scales. For the purpose of choosing the most appropriate tests for Primary Care, we will set an arbitrary time criterion for cognitive test of approximately 10min administration time and we will not describe those that have not been validated in our setting, the characteristics of which can be found in other reviews addressing this issue.13–15 Because of this time criterion, we will also not review short neurophsychological batteries that examine several cognitive domains and that have demonstrated their usefulness for the screening of dementia, such as the 7-min test16 (mean administration time in excess of 10min), the Leganés cognitive test17 (more than 10min), or the ACE-R18,19 (Addenbrooke's Cognitive Examination-Revised, around 20min). These batteries yield more information than short tests and can aid in the differential diagnosis among the different causes of dementia; hence, they are more suited to specialized care clinics. Because they are not used in screening, the short tests that assess behavioural or psychiatric symptoms, for instance the BEHAVE-AD20 or the Neuropsychiatric Inventory (NPI),21 which can be very important in the full evaluation of a patient suspected of having dementia. Most of the tests that will be commented on can be found in monographs about the subject.22–25
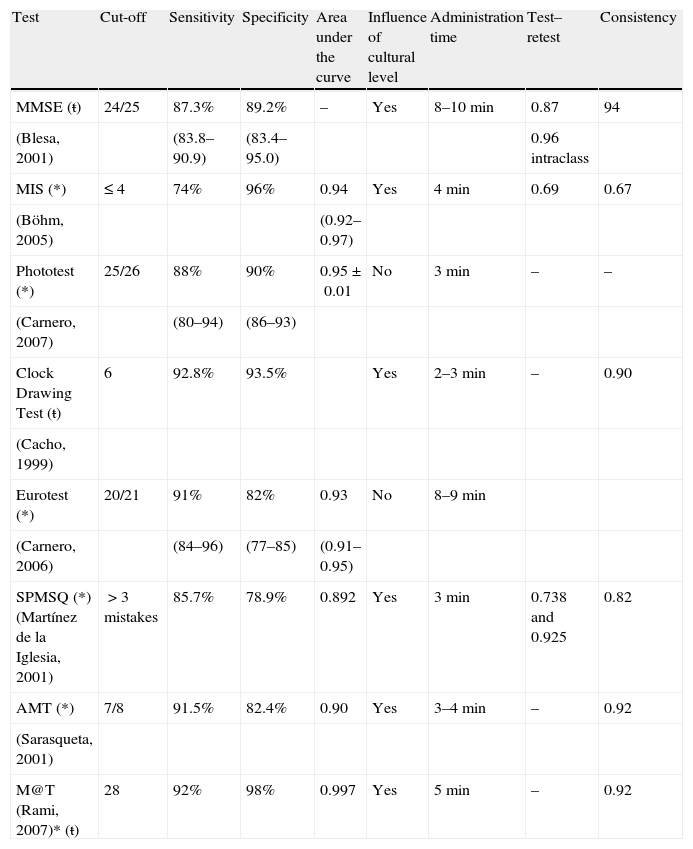
Cognitive testsShort cognitive tests seek to establish an objective measure of the subject's performance on a given task of the examination of the patient's mental status. Therefore, while they are being administered, it is worthwhile to consider them part of a neurological examination as well as to pay attention to subjective aspects that can provide important information about, for example, attention, motivation, the degree of collaboration, the facility to remember instructions, and the time needed to carry it out (more time is almost invariably needed the more severe the cognitive impairment). Table 1 summarizes the main characteristics of these tests.
Screening tests for dementia (*) and/or Alzheimer's disease (ŧ) validated in our setting.
| Test | Cut-off | Sensitivity | Specificity | Area under the curve | Influence of cultural level | Administration time | Test–retest | Consistency |
| MMSE (ŧ) | 24/25 | 87.3% | 89.2% | – | Yes | 8–10min | 0.87 | 94 |
| (Blesa, 2001) | (83.8–90.9) | (83.4–95.0) | 0.96 intraclass | |||||
| MIS (*) | ≤ 4 | 74% | 96% | 0.94 | Yes | 4min | 0.69 | 0.67 |
| (Böhm, 2005) | (0.92–0.97) | |||||||
| Phototest (*) | 25/26 | 88% | 90% | 0.95±0.01 | No | 3min | – | – |
| (Carnero, 2007) | (80–94) | (86–93) | ||||||
| Clock Drawing Test (ŧ) | 6 | 92.8% | 93.5% | Yes | 2–3min | – | 0.90 | |
| (Cacho, 1999) | ||||||||
| Eurotest (*) | 20/21 | 91% | 82% | 0.93 | No | 8–9min | ||
| (Carnero, 2006) | (84–96) | (77–85) | (0.91–0.95) | |||||
| SPMSQ (*) (Martínez de la Iglesia, 2001) | >3 mistakes | 85.7% | 78.9% | 0.892 | Yes | 3min | 0.738 and 0.925 | 0.82 |
| AMT (*) | 7/8 | 91.5% | 82.4% | 0.90 | Yes | 3–4min | – | 0.92 |
| (Sarasqueta, 2001) | ||||||||
| M@T (Rami, 2007)* (ŧ) | 28 | 92% | 98% | 0.997 | Yes | 5min | – | 0.92 |
AMT, Abbreviated Mental Test; MIS, Memory Impairment Screen; MMSE, Mini-Mental State Examination; M@T, Memory Alteration Test; SPMSQ, Short Portable Mental Status Questionnaire.
Since it was first introduced by Folstein et al.26 in 1975, the MMSE has been the most widely used cognitive test in the world and this is probably its greatest advantage, since most of the healthcare personnel involved in evaluating individuals with cognitive impairment are familiar with its use. Moreover, this test has been studied in different populations and for different purposes, such as screening for dementia, to determine its severity, change over time, or response to treatment.27 Another advantage is that it evaluates more cognitive domains than other short tests, specifically, orientation (10 points), registration (3), concentration and calculation (5), recall (3), language (8), and constructional praxis (1). Nevertheless, its disadvantages are also numerous (for instance, it cannot be administered to illiterate individuals), and the person's level of general education is influential, which makes adjustment necessary28 and could decrease its validity.29 It also has the disadvantage of lacking sensitivity to detect initial phases of dementia, since it barely evaluates executive functions, and the measures of visuo-spatial capacity, episodic memory, and semantics are very crude, which takes away from its validity of content. These shortcomings are especially important in subtypes such as fronto-temporal dementia and Lewy body disease. Even in the case of Alzheimer's disease, due to the fact that it contains only 3 items that explore memory, its performance can be poor in the initial stages or in highly educated people. The disturbance alone of these three items has been associated with an increase in the incidence of dementia30 and mortality.31
Administration time, approximately 10min, is variable, which is an issue when using it for screening purposes in overwhelmed Primary Care clinics. In a survey given to physicians in the United States, 58% felt that it took too long to administer.32 Adaptations of the original 30-point version of the MMSE,28,33 as well as versions with slight modifications34–37 have been well studied in our setting by different groups. The only versions that have been authorized by the copyright holder are the original 30-point versions,38 the use of which is more advisable as it enables international comparisons to be more readily made.
Memory Impairment Screen (MIS)The MIS is a short test that evaluates recall of 4 words, both free recall as well as semantic cued recall, with a non-semantic distracting task interposed between encoding and recall. Scoring is calculated with a simple arithmetic operation that gives greater weight to free recall (2×[free recall]+[cued recall]). In its original version in English, validated in a community sample,39 a cut-off of 4 points had 80% sensitivity to detect dementia and 96% specificity. Sensitivity for the diagnosis of Alzheimer's disease was greater (87%), as is logical in a test that only assesses memory. It has been validated in Spain at three units specializing in cognitive impairment,40–42 obtaining results that are similar to those of the original version. Therefore, although it has not been studied in Spain in community or Primary-Care samples, it can a priori be used without problems in these populations. In addition to its validity, similar or superior to that of the MMSE, its main advantages lie in that it is both fast and easy, and its leading disadvantages are that it cannot be administered to illiterate individuals and that it only appraises memory.
PhototestPhototest is a brief diagnostic tool, consisting of three parts: naming of 6 objects, a simple verbal fluency test (male and female proper nouns), and, finally, recalling the 6 initial objects, with both free and semantic cued recall. It is undergoing a strict process of validation: the results of phases I43 and II44 have been published and indicate that it is of use for both the detection of cognitive impairment as well as dementia, with 88% sensitivity and 90% specificity for a cut-off of 25/26. Its primary advantages are ease of use, brevity (close to 3min), and that it is not influenced by level of education and it can be administered to illiterate subjects. As in the case of other very short tests, such as the MIS and the verbal fluency test, its use may be appropriate for screening, but it should be complemented with the use of other tests in order to establish a diagnosis of dementia. The sheets and instructions are available in Spanish at the following website: http://www.fototest.es.
Clock Drawing TestThe Clock Drawing Test is an appealing cognitive task that, in a very short time (2–3min), explores aspects such as attention, remote memory, visuo-spatial ability, visuo-constructional praxis, and executive functions.45 Its brevity and clinical usefulness have led some authors to propose it as an ideal screening method for dementia.46 However, the Clock Drawing Test has two disadvantages for this purpose: on the one hand, it does not assess memory, which makes it inappropriate for the detection of Alzheimer's disease, and on the other hand, it has been poorly accepted and has scant validity in illiterate patients, who are unaccustomed to paper and pencil tasks and who do not perform the test properly. Another issue has to do with the variety of scoring methods, with up to 8 different systems which, despite having a high degree of correlation,47,48 create some confusion among physicians interpreting it. In our setting, the version that is best validated49 has a 92.8% sensitivity rate and a specificity rate of 93.5% for the diagnosis of dementia, with a cut-off of 6 out of 10 in drawing to order. Despite its limitations as a screening test, its qualitative scoring is clinically highly practical in the differential diagnosis of dementia and allows the patient's evolution to be observed.
EurotestThe Eurotest, an update of the coins test,50 is based on the subject's knowledge about legally valid coins and notes. It includes language, memory, calculation, capacity of abstraction and executive function tasks. It was designed with the intention of overcoming linguistic, socio-demographic, or cultural influences, and is appropriate for use with illiterate individuals. It has undergone a strict process of validation with preliminary phases,51 a multicentre, phase-II study,52 and reliability study,53 which have yielded satisfactory results: 91% sensitivity and 82% specificity for a cut-off of 20/21, with a high degree of inter- and intra-rater reliability. Perhaps its greatest limitation is that of the time needed to administer it, approximately 8min, hardly fitting for screening in Primary Care.
Short Portable Mental Status Questionnaire (SPMSQ)Also known as Pfeiffer's test,54 it consists of 10 items that appraise orientation, information, memory, and simple calculation. It is a fast, simple test that can be administered to illiterate patients, although the results are influenced by the person's level of culture; the Spanish version55 is of limited use with a sensitivity rate of 85.9% and specificity of 78.9% for a cut-off of 3 or more mistakes. It is widely used in Primary Care.
Hodkinson's Abbreviated Mental Test (AMT)Hodkinson's test, with its short, 10-point version widely used in the United Kingdom,56 briefly probes orientation, long term memory, and, to a lesser extent, calculation and facial recognition. It is fast and easy to do, but is influenced by the person's level of culture. In our setting, it has been validated by two groups,57,58 with adequate performance in community samples, albeit with a degree of methodological limitation.
Memory Alteration Test (M@T)The Memory Alteration Test is an innovative test that has been designed and validated for the detection of mild, amnesic, cognitive impairment and Alzheimer's disease in the initial phases.59,60 It assesses episodic and semantic verbal memory, whose separate analysis provides addition information, given that altered episodic memory aids in distinguishing subjects with mild amnesic cognitive impairment from controls, whereas semantic memory alterations distinguish mild cognitive impairment from Alzheimer's disease. Among its advantages are the brevity and simplicity of administration, as well as good validity for distinguishing both mild amnesic cognitive impairment as well as Alzheimer's disease. Its usefulness as a screening tool for dementia in general has not been appraised, although it is assumed that it will not be as good, as occurs with other tests that only evaluate memory. Its main contribution is probably the early detection of the mild amnesic cognitive impairment in Primary Care.
Other screening testsVery short tests used in other countries, but those have not been validated in our setting such as the Mini-Cog61 (that examines the recall of three words and drawing a clock), and the GPCOG (General Practitioner Assessment of Cognition)62 have not been included in this review. Both are very short and have demonstrated good discriminant validity. Short tests are also beginning to be designed for the early detection of dementia in special situations, such as in Parkinson's disease.63
Self-reported testsEarly results from the validation of a new test, the TYM (Test Your Memory) have recently been reported.64 The authors administered this test in the waiting rooms at a Memory Unit Clinic in the United Kingdom, without the participation of a healthcare professional. The physician's role was limited to interpreting the results handed in by the subjects. The TYM is a short test (approximately 5min) that examines several cognitive areas and showed better statistical parameters in this study than the MMSE. Undoubtedly, the main advantage of the test is that it does not take any time, although it is not without its disadvantages, such as the influence of the person's level of culture; it cannot be taken by illiterate people; it has a high number of false positive, and it is impossible to determine how much help the patient has received in taking it. Finally, the test is expected to be taken immediately prior to a medical consultation, but the term “self-reported” can lead to confusion and to an indiscriminate use via Internet, where sites are already beginning to appear that offer “the possibility of finding out if you have Alzheimer's”, by means of instruments without any kind of scientific validation.65 A positive result on this type of test can lead to much anguish and give rise to unnecessary consultations. Nonetheless, we are probably facing the first of a series of self-reported cognitive tests. By way of example, an electronic version of the MIS has already been suggested.66
Informant targeted questionnairesIn diagnosing dementia, it is essential to have information provided by a person close to the patient who can describe or confirm the most important clinical traits. Patients are often unaware of their symptoms or minimize them, and it is the relative who can inform as to the true relevance of the problem. For this reason, tests targeting informants yield very useful, complementary information; some of them have even been validated as tools for the screening of dementia, without the use of cognitive tests. In our setting, the one most widely used is the “Test del Informador” (TIN, “Informants’ Test” in Spanish; from the original IQCODE [Informant Questionnaire on Cognitive Decline in the Elderly] in English).67 It comprises a series of questions that are rated from 1 to 5, depending on the current situation versus that of 5 or 10 years earlier. There is a long, 26-question version, as well as a short 17-question versions with greater discriminant power, which is the one that has been validated in the Spanish population.68,69 It has achieved superior diagnostic performance than the MMSE, without being influenced by age, pre-morbid intelligence, or level of culture. Its disadvantage, as with all questionnaires that target people close to the subject, is related to how reliable the informant actually is. Furthermore, the questions and scoring of the answers have a slight difficulty; which means that it should be administered by a professional, with the consequent time involved (approximately 10–15min).
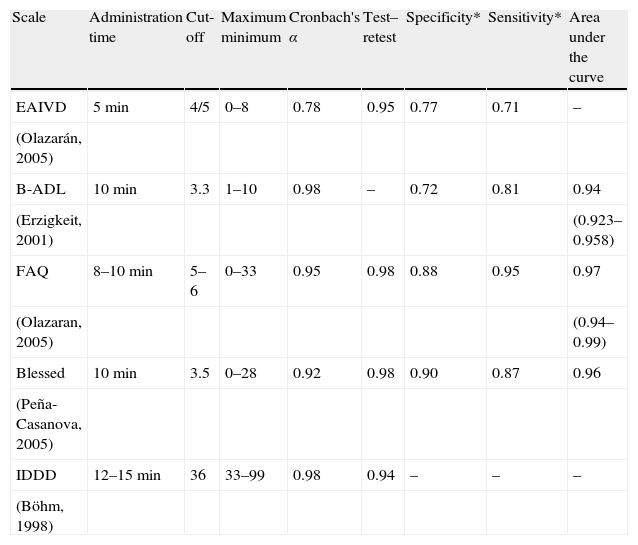
Functional scalesOne of the DSM-IV diagnostic criteria for dementia is that the alteration of cognitive functions must show significant impairment of the individual's social or occupational activity.70 The evaluation of the activities of daily living is a core issue in caring for patients with dementia, both for its diagnosis, as well as in charting its evolution. In this regard, as with the short tests, functional scales can be considered a component of the neurological examinations and represent a structured way to record part of the patient's history. Depending on the type of activities being assessed, functional scales can be divided into basic, instrumental, or advanced. The most useful ones for the early detection of dementia are the Instrumental Activities of Daily Living (IADL), i.e. those activities necessary for the individual to be able to interact in their immediate living arrangement and in their community. They depend on the person's physical ability and, to a large extent, their affective, cognitive, and even social setting.71 They change based on different cultures, communities, and the roles individuals have in their community and are influenced by the subject's gender and age. The most important ones in our setting are: doing housework, cooking meals, using the telephone, using public transportation, managing their own medication, doing laundry or doing the shopping; these IADL are similar across other European countries.72 The most important instrumental scales that have been validated in Spanish are given in Table 2.
Characteristics of the main scales measuring activities of daily living and their performance in the diagnosis of dementia or Alzheimer's disease.
| Scale | Administration time | Cut-off | Maximum minimum | Cronbach's α | Test–retest | Specificity* | Sensitivity* | Area under the curve |
| EAIVD | 5min | 4/5 | 0–8 | 0.78 | 0.95 | 0.77 | 0.71 | – |
| (Olazarán, 2005) | ||||||||
| B-ADL | 10min | 3.3 | 1–10 | 0.98 | – | 0.72 | 0.81 | 0.94 |
| (Erzigkeit, 2001) | (0.923–0.958) | |||||||
| FAQ | 8–10min | 5–6 | 0–33 | 0.95 | 0.98 | 0.88 | 0.95 | 0.97 |
| (Olazaran, 2005) | (0.94–0.99) | |||||||
| Blessed | 10min | 3.5 | 0–28 | 0.92 | 0.98 | 0.90 | 0.87 | 0.96 |
| (Peña-Casanova, 2005) | ||||||||
| IDDD | 12–15min | 36 | 33–99 | 0.98 | 0.94 | – | – | – |
| (Böhm, 1998) |
B-ADL, Bayer Activities of Daily Living Scale; Blessed, Blessed Dementia Scale; EAIVD, Lawton and Brody IADL Scale; FAQ, Pfeffer Functional Activity Questionnaire; IDDD, Interview for Deterioration in Daily Living Activities in Dementia; RDRS-2, Rapid Disability Rating Scale.
The Lawton and Brody IADL Scale was created at the Philadelphia Geriatric Center and appraises 8 areas: ability to use the telephone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medication, and ability to handle finances.73 Each item is rated from 0 to 1, with a minimum score of 0 and a maximum score of 8 (5 in the case of males), depending on the subject's ability. It has good concurrent validity with other scales and with the MMSE. The administration time is short, some 5min. It is administered by professionals, with good reliability and validity for the diagnosis of Alzheimer's disease, especially if an ordinal scoring system is used instead of the dichotic system currently in use.74
Bayer Activities of Daily Living (B-ADL)The B-ADL can be used for the early detection of dementia and consists of 25 questions, five of which measure basic activities, 16 rate instrumental activities, and 4 cognitive function.75 It has been validated by Erzigkeit et al. in Spain and other European countries76 and it takes 10min to administer. In the Spanish study, it showed greater discrimination than the MMSE for the diagnosis of dementias in individuals with a low level of culture and is not influenced by age, education, gender, or country of origin.
Functional Activities QuestionnaireThe Functional Activities Questionnaire examines 11 functional activities (managing money, shopping, preparing beverages and food, information about the neighbourhood, understanding of the means of communication, remembering holidays and birthdays, medication, travelling alone, greeting friends, going out alone) and is scored from 0 (totally able) to 3 (totally unable).77 It takes some 10min to administer. In adapting it to Spanish, a slight modification from the original was introduced.74,78 It is sensitive to changes in mild dementia and shows good sensitivity and specificity.79
Blessed Dementia ScaleThis scale is specific for dementia.80 It covers three areas: changes in the activities of daily living (daily chores, money, remembering lists, finding one's way around at home, outside, in familiar environments, ability to grasp situations, remembering recent events, tendency to dwell in the past), changes in habits (in eating, getting dressed, and control of sphincters), and changes in personality, interests and impulses (growing withdrawal, increased self-centredness, loss of interest in feelings, flat affect, loss of emotional control, inappropriate hilarity, diminished emotional response, sexual indiscretions, lack of interest in hobbies, progressive apathy, unjustified hyperactivity). In general terms, it is easy to administer as it is a semi-structured scale, with an approximate duration of 10min. It has a maximum score of 28 (dependent) and a minimum score 0 (not dependent). It has been validated in Spanish in patients with Alzheimer's disease81; it shows good performance and is not influenced by the patient's level of culture or age.
Interview for Deterioration in Daily Living Activities in Dementia (IDDD)This dementia-specific scale evaluates both instrumental activities, as well as basic activities, and consists of 33 items, 16 of which explore basic activities.82 Its scoring ranges from 33 (no functional deterioration) to 99 (dependent). In Spanish, it has been validated within the NORMACODEM Project,83 as was the previous scale. It has good internal consistency and test–retest reliability and is practical because it evaluates all kinds of ADL, making it a good instrument for the early detection of dementia.
Final conclusions and recommendationsThe short dementia screening tests originate from a very human limitation: lack of time. In a social and health-care environment subjected to growing demands and limited resources, efficiency in the use of physicians’ time has become a necessity. There are numerous tests that have proven to be efficacious in the early detection of dementia and the choice of one over another may be influenced by the work setting and the expertise of each professional. It is advisable that professionals be familiar with a small number of tests so as to become more skilful in their use and to be aware of the advantages and limitations of each one. Based on consultation time and level of care, we put forward the following recommendations:
- 1.
Primary Care Clinics (mean time 5–10min). Two very short tests, the MIS and Phototest, have demonstrated outstanding performance with an administration time of less than 5min, which makes them the most appropriate in our opinion. In an ideal situation, for instance, if support healthcare personnel are available, it would be of great use to associate an informant questionnaire or a functional scale, which would provide complementary information in Primary Care as it would be capable of detecting limitations related to other diseases that the patient suffers. With this evaluation, negative cases can be distinguished from positive or doubtful ones, which would require referral to Specialized Care.
- 2.
Specialized Care Clinics (mean time 10–25min). In this context, short neuropsychological batteries that explore several cognitive areas are more advisable, such as the 7-min test, the ACE-R, or complement the MMSE with tasks that evaluate executive functions, for instance the Clock Drawing Test or phonetic verbal fluency. The recommendation that a functional scale be used is maintained.
- 3.
Specialized Cognitive Impairment Clinics. For typical cases, an evaluation similar to that of Specialized Care may suffice. Doubtful and atypical cases, or those suspected of focal involvement will benefit from a formal neuropsychological examination.
The desired possibility of new treatments appearing in the coming years capable of modifying the course of degenerative disease associated with dementia will make it all the more important for good screening tools to be available.
Conflict of interestsAlberto Villarejo has received fees for academic and consulting activities from Pfizer, Eisai, Novartis, Grunenthal, and Janssen Cilag. Veronica Puertas-Martín has received research aid from Novartis and Lundbeck, Esteve.
Please cite this article as: Villarejo A, Puertas-Martín V. Utilidad de los test breves en el cribado de demencia. Neurología. 2011;26:425–33.