Dear Editor,
Bálint syndrome (BS) is a rare and disabling higher-level visual cognitive impairment consisting of a triad of simultanagnosia (inability to see more than a small number of items simultaneously), optic ataxia (impaired visual guidance of movement of the limbs and body), and oculomotor apraxia (inability to volitionally direct gaze despite the requisite motor substrate) often associated with homonymous lower visual field loss.1,2 It is usually found in lesions involving bilateral parieto-occipital cortices.1–4
We report a novel case of an adult patient who, following an episode of hypoglycemic coma, suffered from difficulty in reaching for objects in the visual field, operating switchboards, understanding the corner of the room and the door, and walking on uneven surfaces, especially using stairs, along with repeated viewing of previous actions. The diagnosis of BS and hallucinatory palinopsia was made after resuscitating the patient.
A 78-year-old man from a low-socioeconomic strata in rural West Bengal, India, was admitted to the emergency department (ED) for acute onset unconsciousness. He was a known diabetic with adequate blood glucose control for the last 5 years on oral antidiabetic drugs (glimepiride 2 mg/day, metformin 2 g/day, and vildagliptin 100 mg/day) and levothyroxine (50 mcg/day) for primary hypothyroidism. He also drank alcohol occasionally. Family members reported that he had a binge drinking last night and did not eat his supper before retiring to bed. At the ED, he was in a coma (Glasgow Coma Scale of 8) and had an alcoholic fetor. His random capillary blood glucose level was 20 mg/dL; other parameters of the complete metabolic panel were reasonably within the normal range. He was then resuscitated with immediate intravenous thiamine 500 mg injection followed rapidly by a 25% dextrose solution infusion. After his blood glucose level stabilized, he was shifted to the ward. He regained some consciousness after 3 days. However, the cloud of confusion took another 3 days to recede. On day 10 of admission, the caregivers complained that though he was conscious, oriented, could recognize and was behaving normally with his visitors, and had intact memory, he had difficulty in reaching for objects in the visual field at a distance, difficulty in gauzing distances, difficulty in reading and operating the switchboard, problems in finding the door of ward toilet, difficulty in walking on uneven surfaces and problems in finding the floor, and problem in especially using stairs. Besides, the patient claimed to visualize short, previously viewed, stereotyped actions, continuously replaying for several minutes for the past 3 days. These incidents occurred minutes after the original visuals and persisted up to 5–10 min, with intact shape, architecture, color, and clarity, suggestive of hallucinatory palinopsia. There was no history of visual snow, micropsia, macropsia, teleopsia, akinetopsia, pelopsia, dysmetropsia, oscillopsia, phosphenes, and photopsias.
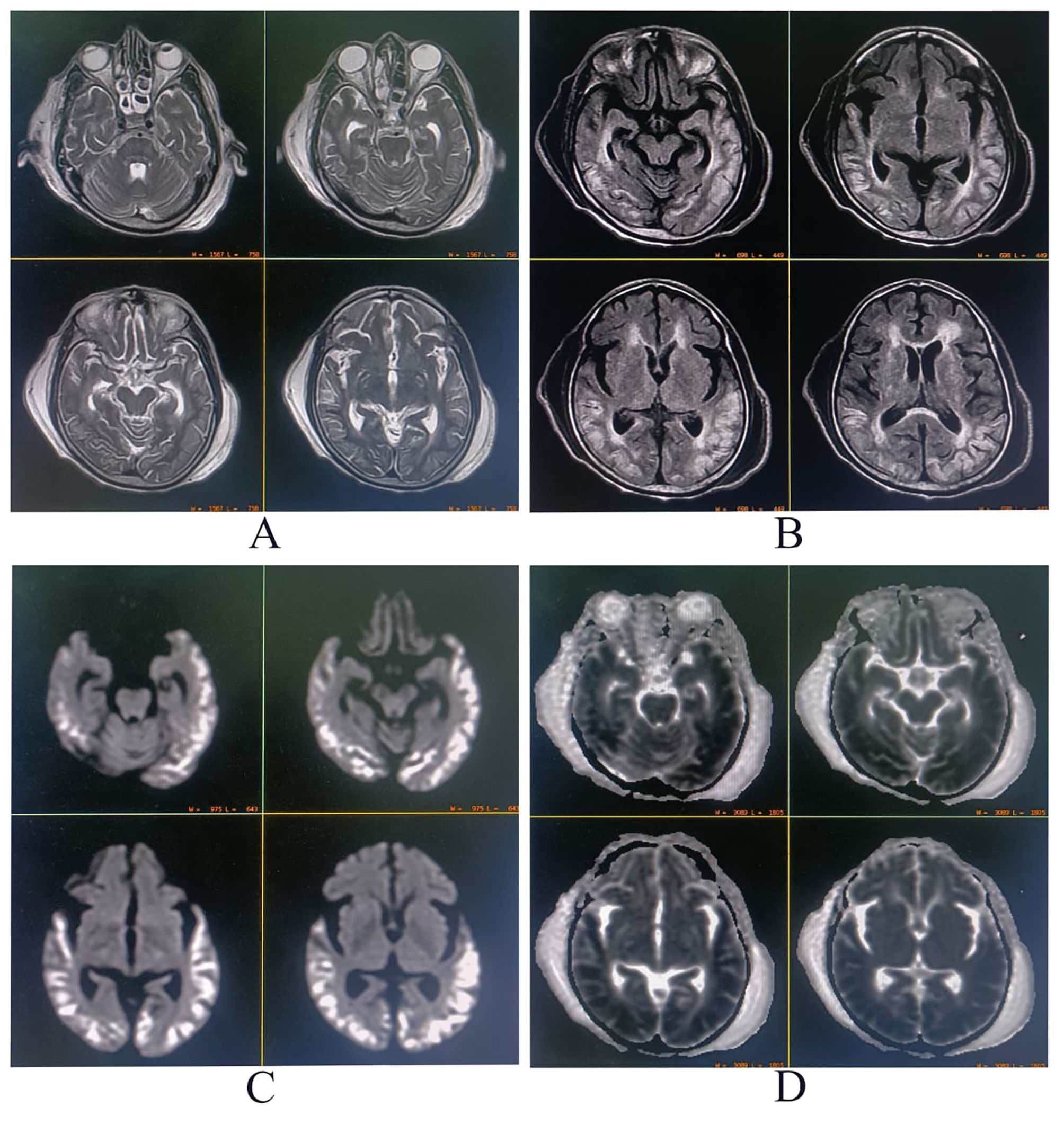
Neurological examination was remarkable for a Mini-mental State Examination of 24/30, fluent speech, and symmetrical lower limb hyperreflexia. Detailed cognitive examination revealed he had higher-order visual and visuospatial dysfunction, including simultanagnosia, optic ataxia, and oculomotor apraxia, i.e., BS. The complete metabolic panel revealed mild normocytic normochromic anemia (hemoglobin 11.8 g/dL), normal electrolytes, liver, kidney, and thyroid function tests, and controlled glycemic status. Serum biomarkers for infection were negative. Serological tests for hepatitis B, C, and HIV were also negative. Brain magnetic resonance imaging (MRI) revealed hyperintensity on T2-weighted and fluid-attenuated inversion recovery sequences with diffusion restriction affecting symmetrically bilateral parietal–occipital–temporal cortex (Fig. 1). Serum thiamine and cyanocobalamin levels were normal. Cerebrospinal fluid examinations were non-contributory (cell count 02 cells, all lymphocytes, protein 35 mg/dL, glucose 75 mg/dL, chloride 115 mEq/L). Anti-thyroid antibodies, autoimmune, and paraneoplastic antibodies panels were negative. Sleep and awake electroencephalogram demonstrated no abnormal discharges nor seizures or status epilepticus. The eventual diagnosis was BS with associated hallucinatory palinopsia following an acute hypoglycemic coma. At 3 months of follow-up, the hallucinatory palinopsia had resolved, but features of BS were only partially corrected (i.e., persistence of oculomotor apraxia and optic ataxia).
Brain magnetic resonance imaging showing signal hyperintensity on T2-weighted (A), fluid-attenuated inversion recovery (B), and diffusion-weighted sequences (C) affecting symmetrically bilateral parietal–occipital–temporal cortex. In contrast, the apparent diffusion coefficient map (D) showed hypointensity in the same distribution.
To our knowledge, this is the first reported case of BS with hallucinatory palinopsia after a hypoglycemic coma, a critical condition characterized by severe hypoglycemia, defined by a plasma glucose level <60 mg/dL (or capillary levels <50 mg/dL) and altered levels of consciousness.5
BS is a visuospatial disorder associated with a triad of optic ataxia, oculomotor apraxia, and simultagnosia classically associated with bilateral inferior parieto-occipital brain injury. However, it has also been reported in diffuse brain injury.1,2 There are only 2 reported cases after hypoglycemic coma, one in an adult patient6 and another in a pediatric patient (without figuring the exalt blood glucose level in the manuscript),7 but both without comprising palinopsia. In the adult case, after intensive visuospatial rehabilitation, his visual symptoms resolved at a 3-year follow-up, but with persistent higher-level cognitive deficits and frontal symptoms.6
Palinopsia is an unusual symptom of hypoglycemic patients. When it is secondary to structural disease, it usually appears in patients with impaired vision who are not entirely blind and transiently during the progression or resolution of visual field defect and usually disappears in a few days.8,9 According to the Bayesian theory, which states that surrounding images can be predicted using stored memories of previous images, palinopsia could be due to a mismatch in the brain's prediction phase.8 The pathophysiology of palinopsia is unknown, but one hypothesis suggests a local irritation of the parieto-occipital cortex by surrounding edema,8,9 like the cytotoxic edema following hypoglycemic coma as occurred in our case. Hallucinatory palinopsia, encompassing categorical incorporation, scene preservation, and formed image preservation, indicates visual memory encoding, processing, or retrieval dysfunction.9 It can originate from bilateral parietal, temporal, and occipital cortice lesions, for instance, because of COVID-19-induced posterior reversible encephalopathy syndrome.9
Palinopsia has been linked to a wide variety of etiologies and mechanisms, such as drug-induced, idiopathic seizures, migraine, psychiatric conditions, metabolic disturbances, head trauma, and structural brain lesions, mainly those involving parietal and parieto-occipital connections.10 The high-resolution afterimages that are long-lasting, isochromatic, and unaffected by environmental conditioning and motion are typical of hallucinatory palinopsia, a subtype of palinopsia that is characterized by a dysfunction in visual memory and is produced by posterior cortical lesions and/or seizures.10 Conversely, illusory palinopsia represents after images that are unformed, indistinct, or of low resolution and are affected by environmental conditioning. A dysfunction in visual perception causes this category of palinopsia and is due to migraines, prescription drugs, illicit drugs, and/or head trauma.10
Finally, hypoglycemic encephalopathy includes a broad clinical spectrum and may be expressed as seizures, focal neurological deficits, and/or decreased level of consciousness. It is essential to rule out toxic and metabolic causes. Regarding neuroimaging, diffusion-weighted brain MRI sequences show hyperintensities in the gray matter of the cortex, hippocampus, internal capsule, and basal ganglia in up to 70% of cases,11 and thalamic indemnity is a characteristic feature,12 unlike hypoxic encephalopathy.12 The brain's vulnerability to hypoglycemia varies between areas of the cerebral cortex, being the parietal occipital cortex the most prone to be affected.13–15 Despite these brain areas' differences in vulnerability, a more significant lesion extension on MR images seems to predict higher morbidity and mortality. Then, neuroimaging is an essential tool not only for diagnosis but also for neurological prognosis.11
This case highlights the importance of including hypoglycemic coma in the etiologies of this higher-order visual syndrome and differentiating it from non-convulsive status epilepticus, hypoxic encephalopathy, and anti-thyroid peroxidase (anti-TPO)/anti-thyroglobulin (anti-TG) antibody-related neurologic disorders responsive to steroids (ATANDS), among others, since the treatment is entirely different and affordable. Early glycemic correction is of utmost importance in hypoglycemic coma cases.
However, that is not the sole reason for the publication of the case. Instead, the authors have experienced cases of higher-level visual cognitive dysfunctions like BS (complete or partial) and hallucinatory palinopsia, most often not even diagnosed. Worst, in some cases, this gets misdiagnosed as functional blindness, psychotic syndromes, or even malingering because of the unfamiliarity of primary-care physicians with this entity, lack of precise perception about higher-order visual cognitive symptoms, and patient's inability to describe the problem. Most of the time, clinicians reach a retrospective diagnosis after viewing the neuroimaging.
Study fundingNil.
DisclosuresDr. Ritwik Ghosh (ritwikmed2014@gmail.com) reports no relevant disclosures.
Dr. Moisés León-Ruiz (pistolpete271285@hotmail.com) reports no relevant disclosures.
Dr. Souvik Dubey (drsouvik79@gmail.com) reports no relevant disclosures.
Dr. Julián Benito-León (jbenitol67@gmail.com) reports no relevant disclosures.
Ethics statementWritten informed consent was obtained from the patient to publish this case report and any accompanying images.
Author contributionsAll authors contributed significantly to the creation of this manuscript; each fulfilled criterion as established by the ICMJE.
J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).