Dear Editor:
Guillain-Barré syndrome (GBS) is a polyradiculoneuropathy manifesting as areflexic flaccid paralysis. Onset is usually in the lower limbs; paralysis may progress centripetally; and some patients develop respiratory failure, with 30% requiring orotracheal intubation. The syndrome is classified as an autoimmune disorder. Seventy percent of patients present a trigger factor 2 weeks prior to symptom onset; the trigger is generally a respiratory or gastrointestinal infection, with the most frequent pathogens being Campylobacter jejuni,1 cytomegalovirus,2 Epstein–Barr virus, and Mycoplasma pneumoniae, and more recently SARS-CoV-2.3 GBS has also been reported after vaccination against influenza, rabies, measles, and SARS-CoV-2.
The classical form of GBS is acute demyelinating polyradiculoneuropathy, which is classified into several types, including acute motor axonal neuropathy (AMAN), acute motor-sensory axonal neuropathy (AMSAN), and Miller-Fisher syndrome, which presents with ophthalmoplegia, ataxia, and areflexia. GBS is diagnosed with electroneurography study and cerebrospinal fluid (CSF) analysis, which typically reveals albuminocytologic dissociation.
We present the case of an 83-year-old woman with personal history of essential hypertension, hypercholesterolaemia, hypothyroidism, glaucoma, and carpal tunnel syndrome. She was under treatment with levothyroxine (25 μg/day), lormetazepam (1 mg/day), losartan (50 mg/day), naproxen (500 mg as needed), omeprazole (20 mg/day), and paracetamol (1 g as needed).
She received the first dose of the Pfizer SARS-CoV-2 vaccine (batch ER9470) 36 days before symptom onset, and the second dose (batch EW6326) 13 days prior to onset.
She consulted due to 48 h' progression of instability, disorientation, difficulty swallowing, and lower limb weakness, and had been unable to walk for 24 h prior to consultation. Physical examination revealed tachypnoea and grade III/VI systolic murmur apparent at the mitral area; the patient was stuporous with spontaneous opening of the eyes; she responded to painful stimuli with flexion of the lower limbs; plantar reflex was flexor bilaterally; and pharyngeal reflex was diminished.
A complete blood count revealed a leukocyte count of 27.92 × 109 cells/L (normal range, 4.80–10.80), haemoglobin level of 11.0 g/dL (12.0–16.0), and platelet count of 79 × 109/L (140–450). Haemostasis was normal, with a D-dimer level of 14,690 ng/mL (0–243), slightly prolonged prothrombin time, a deficit of factors II and VII, and low ATIII level. The polyspecific direct Coombs test was positive, direct Coombs test for IgG was weakly positive, and direct Coombs test for C3d and anti-erythrocyte antibodies was negative. Biochemistry tests revealed a urea level of 179 mg/dL (19–62), creatinine level of 1.43 mg/dL (0.51–0.97), glomerular filtration rate (estimated with the CKD-EPI equation) of 33.89 mL/min/1.73 m2, GOT/AST of 50 U/L (3–35), GPT/ALT of 32 U/L (3–35), serum GGT level of 186 U/L (4–38), serum alkaline phosphatase of 253 U/L (35–110), and total bilirubin of 1.54 mg/dL (0.30–1.20). Ferritin level was 2385.7 ng/mL (30.0–300.0). C-reactive protein level was 290.7 mg/L (0.2–5.0). Glucose, uric acid, calcium, phosphate, CK, sodium, potassium, cholesterol, triglycerides, and glycated haemoglobin values were normal. Total protein test detected a polyclonal increase in gammaglobulins. Total serum protein level was 6.28 g/dL (6.60–8.30) and serum albumin level was 2.0 g/dL (3.4–5.2). Immunoglobulin testing revealed an IgG level of 22.32 g/L (7.5–16), IgM level of 6.57 g/L (0.46–3.04), and IgG1 level of 17.06 g/L (4.9–11.4); IgA, IgG2, IgG3, and IgG4 levels were normal. TSH levels were normal. Vitamin D (25-hydroxycholecalciferol) level was 9.7 ng/mL (>30); levels of vitamins B1, B6, B9, B12, and E were normal; magnesium, zinc, cobalt, mercury, nickel, selenium, silver, copper, chromium, and tin levels were also normal. HPLC detected normal homocysteine levels. Blood and urine porphyrin levels were normal. Amyloid A level was 6.7 mg/L (0–5.0).
A tumour marker study revealed CA 125 level of 89.9 IU/mL (normal range, 1.0–35.0), Cyfra 21-1 of 3.97 ng/mL (0.1–3.3), and neuron-specific enolase of 24.7 ng/mL (0–18.3). Serum beta-2-microglobulin level was 6.3 mg/L (0.4–3.0) and serum chromogranin A level was 520 ng/mL (0–100). Testing for squamous cell carcinoma antigen, CA 19.9, CEA, CA 15-3, and CA 72-4, and the Bence-Jones protein urine test returned negative results.
Blood and urine cultures also yielded negative results. Blood serology results were negative for Legionella pneumophila, M. pneumoniae, Borrelia burgdorferi, Brucella, Treponema pallidum, Toxoplasma gondii, cytomegalovirus, Epstein–Barr virus, varicella zoster virus, herpes simplex virus 1 and 2, parvovirus B19, hepatitis B and C viruses, and HIV. CLIA serology testing returned positive results for SARS-CoV-2 IgG antibodies (13.1 AU/mL).
Results for lupus anticoagulant were positive, with anticardiolipin IgM antibody level of 55 MPL (normal range, 0–10), beta-2 glycoprotein IgM level of 11 U/mL (0–10), aldolase of 14.2 U/L (1.2–7.6), and anti-TPO antibodies (microsomal) of 58.0 U/mL (0.0–9.0). Results were negative for antinuclear antibodies, ADA, ECA, rheumatoid factor, anti-citrullinated peptide antibodies, anti-neutrophil cytoplasmic antibodies, anti-transglutaminase IgA, anti-Ro (SSA) antibodies, serum anti-myositis antibodies (Mi-2 alpha, Mi-2 beta, TIF1 gamma, MDA5, NXP2, SAE1, Ku, PM/Scl-100, PM/Scl-75, Jo-1, SRP, PL-7, PL-12, EJ, OJ, and Ro-52), Scl-70, CENP-A, CENP-B, RNA pol III (RP11), RNA pol III (RP155), fibrillarin, NOR90, Th/To, PDGFR, component C3, component C4, and haemolytic component CH50. IL-6 level was 32.0 pg/mL (5.3–7.5); IL-1B level was normal.
Serum antiganglioside IgM antibody determination revealed a band corresponding to anti-GM1. Testing for antineuronal antibodies (amphiphysin, CV2, PNMA2 [Ma2/Ta], Ri, Yo, Hu, recoverin, SOX1, titin, Zic4, GAD65, and Tr [DNER]) in serum yielded negative results. Serology testing for autoimmune encephalopathies returned negative results for anti-HMGCR, anti-nodal/paranodal, anti-ganglioside IgG, and anti-MAG antibodies.
CSF was clear, and presented a protein level of 84 mg/dL (normal range, 15.00-45.00), leukocyte count of 9 cells/mm3, no red blood cells, normal glucose level, negative ADA, and normal albumin level; CSF culture results were negative; CSF serology for antineuronal antibodies (amphiphysin, CV2, PNMA2 [Ma2/Ta], Ri, Yo, Hu, recoverin, SOX1, titin, Zic4, GAD65, and Tr [DNER]) were negative; autoimmune encephalopathy screening yielded negative results for antibodies against the NMDA, AMPA1, AMPA2, and GABA receptors; cytology results for T. pallidum, Brucella, T. gondii, rubella, cytomegalovirus, varicella zoster virus, herpes simplex 1 and 2 viruses, and parvovirus B19, hepatitis C and PCR results for cytomegalovirus and varicella zoster virus were negative.
Electrocardiography identified rapid atrial fibrillation. A CT scan revealed mild pericardial effusion and pleural effusion. Brain MRI identified acute/subacute ischaemic lesions in multiple territories (left cerebellar hemisphere, dorsomedial nucleus of the left thalamus, and bilateral supratentorial white matter), with signs of haemorrhagic transformation of the lesion in the right centrum semiovale. A cervicothoracolumbar spinal MRI scan revealed degenerative disc disease at C3–C4 and C6–C7, with no impact on the spinal cord or nerve roots; lumbar spondyloarthrosis; and normal spinal cord morphology and signal intensity. A transthoracic echocardiography study detected aortic valve endocarditis. Electromyography revealed severe AMAN (Fig. 1). Electroencephalography revealed slow, poorly integrated background activity with generalised irritative-lesional elements in response to stimuli and upon waking.
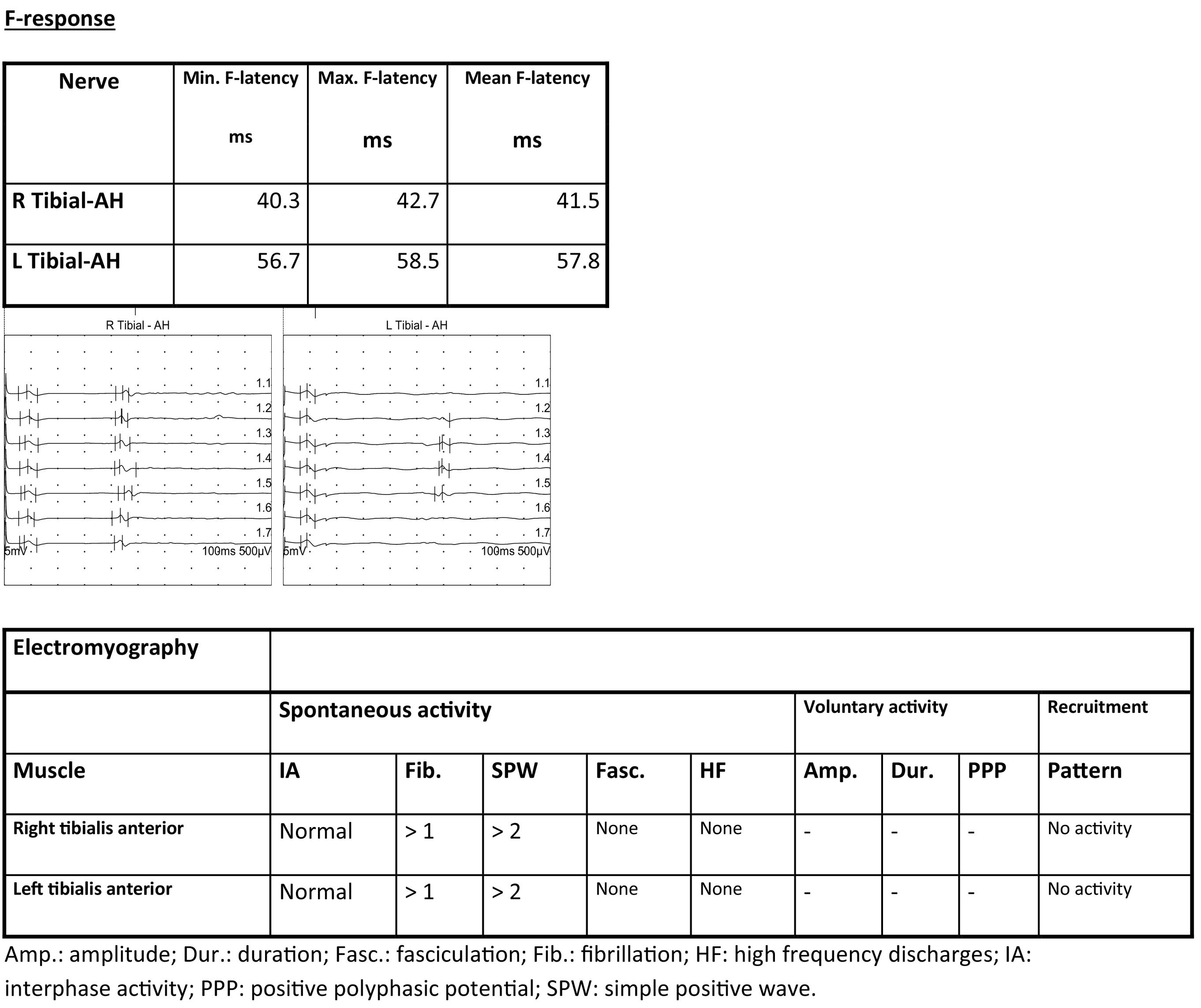
Reduced amplitude was observed in both internal popliteal nerves, with preserved conduction velocity. Surface electrodes detected no response in either external popliteal nerve. F-response latency was prolonged in the left internal popliteal nerve.
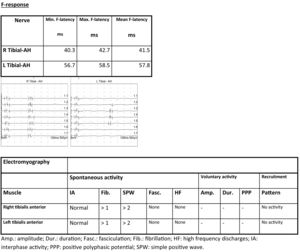
EMG showed signs of denervation in both tibialis anterior muscles. The patient displayed an effort pattern with inability to recruit motor unit potentials.
GBS was treated with corticosteroids and gammaglobulins; the patient did not recover lower limb mobility and required enteral nutrition with percutaneous endoscopic gastrostomy.
The ischaemic infarcts may have been secondary to the atrial fibrillation and the coagulopathy demonstrated by the high D-dimer levels.
Endocarditis was treated with triple antibiotic therapy, and progressed favourably.
GBS is an acute autoimmune polyradiculoneuropathy, associated with a trigger factor 2 weeks prior in 70% of cases; the trigger will generally be respiratory or gastrointestinal infection with a bacteria or virus, including SARS-CoV-2.4
The syndrome has also been associated with vaccination against SARS-CoV-2, for instance with the vaccines developed by Oxford-AstraZeneca and Pfizer-BioNTech.5–7 Our patient presented symptoms 13 days after receiving the second dose of the vaccine, and presented negative results in blood cultures and serology studies for the most common infections.
Eighty percent of CSF proteins reach the CSF by passive diffusion from the plasma and the remaining 20% are generated by intrathecal synthesis. Acute stress increases the permeability of the blood–brain barrier (BBB).8–10 Our patient presented elevated CSF protein levels with a normal cell count; serum protein levels were slightly below normal values. The liver is the only organ to synthesise albumin; therefore, all the albumin in the CSF originates in the plasma. Our patient presented low levels of the protein in both the serum and the plasma. Patients with sepsis may present increased levels of TNF alpha, potentially inducing production of IL-6 and IL-6B, which may increase the permeability of the BBB11,12; our patient presented elevated levels of IL-6. The BBB may also be altered as a result of brain ischaemia11; in our patient, brain MRI revealed acute/subacute lesions in multiple territories. Serology and PCR results for the most frequent viruses were negative.
AMAN is considered to be an autoimmune process with alterations to the blood–nerve barrier, causing degeneration of motor axons. Studies report a similarity between lipopolysaccharides on the external aspect of the cell wall of C. jejuni and GM gangliosides13 at or near the nodes of Ranvier, which would explain why antibodies targeting these cell wall polysaccharides would present cross-reactivity against carbohydrates present in nerve gangliosides. Our patient presented anti-GM1 antibodies, although no pathogen was identified. However, according to the literature, these antibodies are associated with C. jejuni gastroenteritis in 76% of cases. The spinal cord MRI study identified no pathological changes that may explain the elevated CSF protein level.
As cancer was ruled out, the elevated levels of amyloid A, CA 125, neuron-specific enolase, and beta-2 microglobulin in the serum would be explained by acute-phase reactants.
The patient presented endocarditis, which was treated with antibiotics, with a good response. To date, vaccines against SARS-CoV-2 have not been associated with increased prevalence of bacterial infections.
This is the first study to describe AMAN-type GBS after administration of the second dose of the Pfizer SARS-CoV-2 vaccine.
Ethical considerationsThe authors observed their centre's protocols for the publication of patient data. The patient gave informed consent for the publication of this case report.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis study has received no funding of any kind.