Dear Editor:
Medication persistence is defined as the time of continuous therapy, from initiation of treatment to its discontinuation.1 Factors conditioning medication persistence include treatment effectiveness, complexity of dosage, adverse effects, impact on patient lifestyle, patient motivation, quality of the information provided by the healthcare staff on the condition, and need for treatment after onset.2,3 Lack of adherence to and persistence of treatments prescribed by healthcare professionals translates into reduced treatment effectiveness and increased healthcare costs.
Several studies using data from medical records and databases have assessed the persistence of treatments in multiple sclerosis (MS), and generally conclude that oral treatments are more persistent than injectable treatments.4,5 These real-world studies also report lower persistence than that described in clinical trials, undoubtedly due to differences between our patients and those included in clinical trials; persistence is largely influenced by the presence of various comorbidities in real-life patients.
The aim of our study was to determine the persistence of first-line oral treatments for MS, teriflunomide (TERI) or dimethyl fumarate (DMF), and the reasons for discontinuation in a hospital series. The study was approved by the research ethics committee of the Galicia-Sur Health Research Institute.
We requested that our hospital’s pharmacy department provide access to the registry of patients who started treatment with TERI or DMF for MS between 1 June 2015 and 31 October 2021. We identified 125 patients who started treatment with TERI and 77 patients with DMF. The baseline characteristics of each group are shown in Table 1. Statistical analysis was performed using the SPSS statistics software. P-values < .05 were considered statistically significant. Medication persistence was estimated using Kaplan-Meier survival curves, which were compared with the Mantel-Cox test (log rank test).
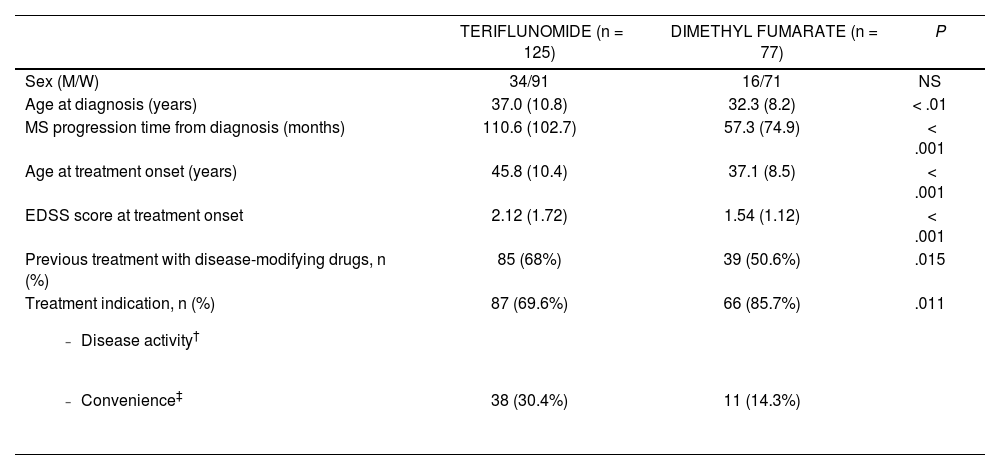
Baseline characteristics of the treatment groups.
| TERIFLUNOMIDE (n = 125) | DIMETHYL FUMARATE (n = 77) | P | |
|---|---|---|---|
| Sex (M/W) | 34/91 | 16/71 | NS |
| Age at diagnosis (years) | 37.0 (10.8) | 32.3 (8.2) | < .01 |
| MS progression time from diagnosis (months) | 110.6 (102.7) | 57.3 (74.9) | < .001 |
| Age at treatment onset (years) | 45.8 (10.4) | 37.1 (8.5) | < .001 |
| EDSS score at treatment onset | 2.12 (1.72) | 1.54 (1.12) | < .001 |
| Previous treatment with disease-modifying drugs, n (%) | 85 (68%) | 39 (50.6%) | .015 |
Treatment indication, n (%)
| 87 (69.6%) | 66 (85.7%) | .011 |
| 38 (30.4%) | 11 (14.3%) |
Data are expressed as mean (standard deviation), unless other wise indicated.
EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging; MS: Multiple sclerosis; NS: not significant.
Of the patients receiving TERI, 39 (31.2%) discontinued treatment; disease activity (relapse, radiological activity, or progression) was the reason for discontinuation in 21.6% of cases, and adverse reactions in 8%. Of the patients receiving DMF, 29 (37.7%) discontinued treatment; the reason for discontinuation was clinical/radiological activity in 7.8% of cases and adverse reactions in 26%. The difference in the reasons for discontinuation between groups was statistically significant (P < .01), with disease activity being the most frequent reason in the TERI group and presence of adverse reactions being more common in the DMF group. Fig. 1 shows the Kaplan-Meier survival curve analysing medication persistence with TERI and DMF. At 60 months, persistence in the TERI group (61%) is slightly higher than in the DMF group (55%), but the difference was not statistically significant (P = .13). One limitation of this real-world study is that the 2 treatment groups presented different baseline characteristics.
Lymphocytopaenia was the reason for discontinuation in up to 50% of patients discontinuing treatment due to adverse reactions to DMF, but was not recorded in any patient receiving TERI. This differential profile between TERI and DMF has led us to consider TERI as the ideal treatment for switching from injectable drugs to oral drugs for the convenience of patients who have remained clinically and radiologically stable, in whom safety and tolerability take priority over anti-inflammatory power due to the low level of inflammatory activity observed in these patients.6 Another characteristic to be considered when switching drugs is the potential effect of TERI in slowing brain atrophy.7,8 Likewise, patients presenting greater clinical/radiological activity may be treated with DMF, as demonstrated by propensity score studies that support the effectiveness of DMF over TERI.9,10
Persistence is a complex concept involving effectiveness, safety, and tolerability of treatment as well as patient satisfaction, and should be considered a measure of treatment efficiency.
Sources of funding- -
None.
Dr García Estévez has received fees from Sanofi in relation to product presentations (teriflunomide) and from Biogen for participating in an advisory board (dimethyl fumarate).