Biomaterial and stem cell -based treatment strategies for diseases of the central nervous system
Más datosCell therapy and tissue engineering are promising therapeutic strategies for such neurodegenerative diseases as amyotrophic lateral sclerosis (ALS). Studies of different cell types have reported promising yet contradictory results, and the development of biomaterials enabling the integration of exogenous and host cells has been slow. This review analyses the most promising results published to date and emphasises the need for further research into the clinical application of biomaterials.
La terapia celular y la ingeniería tisular han dado un paso adelante en el desarrollo de posibles estrategias terapéuticas en enfermedades neurodegenerativas como la Esclerosis Lateral amiotrófica (ELA). Diversos tipos celulares se han ensayado tanto con resultados prometedores como contradictorios, por su parte, el desarrollo de biomateriales que favorezcan la integración de células exógenas en el huésped ha sido lento. La presente revisión pretende, abordar los resultados más prometedores y recordar el camino que aún hay por recorrer en la traslación a la clínica.
Amyotrophic lateral sclerosis (ELA) is a neurodegenerative disease affecting motor neurons in the brain, brainstem, and spinal cord. It is characterised by muscle weakness and atrophy, fasciculations, spasticity, and paralysis. The average life expectancy after symptom onset is 3–5 years (1). Authorised pharmacological treatments for ALS include riluzole (a glutamate release inhibitor) and edaravone (a free radical scavenger); however, these medications only slow disease progression and have limited efficacy (2).
Over the past 2 decades, multipotent stem cell–based therapies for the treatment of such neurodegenerative and vascular diseases as stroke, Parkinson's disease, and ALS have sparked interest in promoting the restoration and regeneration of the nervous system (3). Due to the pathophysiological characteristics of the disease and the lack of a curative treatment, several research groups have focused on the development of cell therapies and the search for vehicles that promote survival of the implanted cells in a hostile environment (4). These vehicles are biocompatible natural or synthetic materials that encapsulate cells or create a scaffold that promotes the integration of the cells into the host tissue. These cells would promote modulation of the inflammation, restoration, and endogenous repair; research is currently focused on ensuring survival of the implanted cells (5–7).
Mesenchymal stem cells: A promising alternativeMesenchymal stem cells (MSC) were first described by Friedenstein et al.(8) in 1970, in a study using bone marrow mononuclear cells as adherent clonogenic cells. They are considered fibroblast colony–forming units, and present a high in vitro replication capacity.
MSCs can be isolated from a variety of tissues, including bone marrow, muscle tissue, adipose tissue, skin, cartilage, blood vessels, menstrual blood, and tooth pulp. These cells acquire a fibroblast-like appearance, expressing such markers as CD105, CD73, and CD90; however, they do not express CD45, CD34, or CD14 (9,10).
No cases of such adverse events as teratomas have been reported with MSC therapy; these cells are therefore considered to be safe, with the added benefit that they do not present the ethical limitations associated with embryonic or fetal stem cells. MSCs present the basic properties of stem cells: self-renewal, multilineage differentiation potential, clonality, and capability to regenerate tissue in vivo. Therefore, they are considered to be multipotent cells, as they can differentiate into several mesenchymal lineages, such as those found in bone, cartilage, adipose, and muscle tissue (9,10).
The therapeutic potential of MSCs is linked to their immunoregulatory paracrine activity, with the secretion of soluble factors (secretomes), including, among others:
• Growth factors: vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), and hepatocyte growth factor (HGF) (11).
• Anti-inflammatory factors: interleukin-10 (IL-10), transforming growth factor beta 1 (TGF-β1), and mechanisms linked to exosome and mRNA release (12,13).
Over the last 20 years, attempts have been made to extrapolate results from animal studies to human disease (14). Animal studies have yielded promising results. For example, transgenic mutant SOD1 mice have been administered MSCs via intrathecal or intravenous injection, or a combination of the 2 routes; the procedure has been found to be safe and to effectively delay motor impairment, reduce inflammation, and promote the secretion of cytokines and growth factors that increase cell survival, allowing animals to live longer (15,16).
Mesenchymal stem cells for the treatment of neurological diseasesMSCs are promising candidates for regenerative medicine thanks to their paracrine properties (10). When nervous tissue is damaged, the accumulation of inflammatory cells, fibroblasts, and astrocytes causes MSCs to secrete a variety of factors, including glial cell line–derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), VEGF, IGF-1, nerve growth factor (NGF), ciliary neurotrophic factor (CNTF), and neurotrophin 3 (NT-3), in order to promote motor neuron survival. The paracrine action of these factors protects nervous tissue from fibrosis, apoptosis, and oxidative stress, promotes angiogenesis, and has immunomodulatory and neuroprotective effects (17,18).
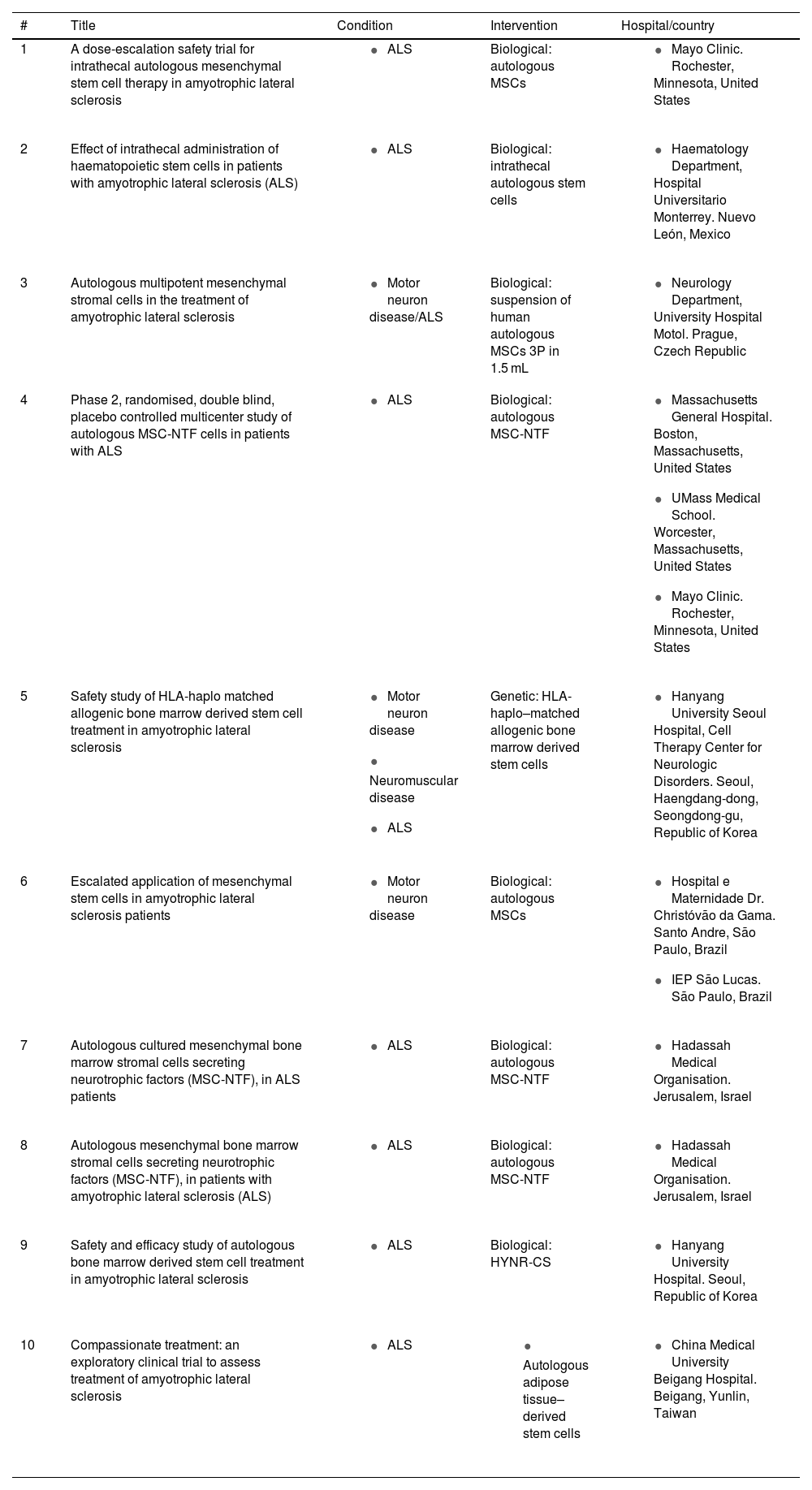
Clinical trialsClinical trials using MSCs (both adipose tissue– and bone marrow–derived) to treat patients with ALS have provided useful information about the safety and efficacy of this therapy (19). Other studies have evaluated the administration route (intravenous, intrathecal, or intramuscular) and dosing (single dose or repeated doses) over variable follow-up periods. A total of 93 clinical trials have been completed to date, providing data of considerable clinical interest; table 1 shows the 10 most recently concluded clinical trials providing satisfactory results (see Table 1). Different research groups report a lack of adverse reactions and improvements in quality of life, mainly due to delays in the need for gastrostomy tube feeding/parenteral nutrition and respiratory support and to longer patient survival.
The 10 most recent completed clinical trials, from a total of 93 trials published on clinicaltrials.gov.
| # | Title | Condition | Intervention | Hospital/country |
|---|---|---|---|---|
| 1 | A dose-escalation safety trial for intrathecal autologous mesenchymal stem cell therapy in amyotrophic lateral sclerosis |
| Biological: autologous MSCs |
|
| 2 | Effect of intrathecal administration of haematopoietic stem cells in patients with amyotrophic lateral sclerosis (ALS) |
| Biological: intrathecal autologous stem cells |
|
| 3 | Autologous multipotent mesenchymal stromal cells in the treatment of amyotrophic lateral sclerosis |
| Biological: suspension of human autologous MSCs 3P in 1.5 mL |
|
| 4 | Phase 2, randomised, double blind, placebo controlled multicenter study of autologous MSC-NTF cells in patients with ALS |
| Biological: autologous MSC-NTF |
|
| 5 | Safety study of HLA-haplo matched allogenic bone marrow derived stem cell treatment in amyotrophic lateral sclerosis |
| Genetic: HLA-haplo–matched allogenic bone marrow derived stem cells |
|
| 6 | Escalated application of mesenchymal stem cells in amyotrophic lateral sclerosis patients |
| Biological: autologous MSCs |
|
| 7 | Autologous cultured mesenchymal bone marrow stromal cells secreting neurotrophic factors (MSC-NTF), in ALS patients |
| Biological: autologous MSC-NTF |
|
| 8 | Autologous mesenchymal bone marrow stromal cells secreting neurotrophic factors (MSC-NTF), in patients with amyotrophic lateral sclerosis (ALS) |
| Biological: autologous MSC-NTF |
|
| 9 | Safety and efficacy study of autologous bone marrow derived stem cell treatment in amyotrophic lateral sclerosis |
| Biological: HYNR-CS |
|
| 10 | Compassionate treatment: an exploratory clinical trial to assess treatment of amyotrophic lateral sclerosis |
|
|
|
https://clinicaltrials.gov/ct2/results?term=cell+therapy&cond=ALS&Search=Apply&recrs=e&age_v=&gndr=&type=&rslt=
It should be noted that only one clinical trial reported adverse reactions, and was therefore suspended. This trial evaluated the safety and efficacy of infusion of autologous bone marrow-derived MSCs in 6 patients, and reported neurological symptoms not attributable to the disease (ClinicalTrials.gov identifier: NCT01082653).
ALS: amyotrophic lateral sclerosis; HYNR-CS: bone marrow-derived mesenchymal stem cells; MSC: mesenchymal stem cell; MSC-NTF: mesenchymal stem cells secreting neurotrophic factors.
Interestingly, and despite their limited results, these trials use electromyography to guarantee that intrathecal injection does not cause adverse effects due to displacement of the spinal cord parenchyma (20,21,22).
All the clinical trials completed to date used MSCs. Their results consistently show that (23,24):
1. MSC therapy is safe and viable, despite the small number of participants included.
2. MSC therapy achieves a transient delay in the natural course of the disease.
3. Neurotrophic factors play a major role in neuronal survival and represent a promising therapeutic strategy for ALS.
Biomaterials and stem cells in amyotrophic lateral sclerosis
No clinical trial of cell therapy and biomaterials for the treatment of ALS is currently underway. All studies are still in the preclinical phase (both in vitro and in vivo studies) (25,26); their primary objective is to generate a suitable microenvironment enabling the implanted cells to survive the adverse conditions caused by the disease. One example is the study by Kalkowski et al., (27) who used a sodium alginate–based hydrogel rich in Mn2+ ions, which promotes a favourable environment for the implanted cells and constitutes an excellent contrast medium for MRI guidance since it produces a strong T1 signal. Vieira et al. (28) used methacrylated gellan gum and hyaluronic acid hydrogel blends as a culture medium for the cell suspension and subsequently injected cells intrathecally, obtaining similar results to those reported for sodium alginate–based hydrogel.
Cell therapy tourism
The results of previous studies into cell therapy for ALS are controversial (29). However, clinics in different countries across Europe, Asia, and Latin America have been reported to advertise “miracle” treatments for severe illnesses. These clinics purport to cure patients with stem cell–based therapy. They operate on the basis of controversial legislation allowing the use of cell therapies whose efficacy has not been confirmed by studies complying with such international regulatory bodies as the United States Food and Drug Administration or the European Medicines Agency, or following the International Society for Stem Cell Research guidelines (30).
ConclusionsCell therapy has emerged as a promising treatment strategy for ALS, and constant advances are being made in the use of biomaterials that enhance the benefits of stem cells. However, much remains to be understood. Further research in the form of basic studies and well-designed clinical trials following the applicable regulations may shed light on these promising therapeutic strategies.
Conflicts of interestThe authors have no conflicts of interest to declare.