Cognitive impairment, usually associated with seizures and psychosis, is one of the main symptoms of autoimmune encephalitis. The objective of our study is to analyse cognitive impairment in patients with autoimmune encephalitis.
MethodsWe conducted a descriptive cross-sectional study of clinical data from 39 patients older than 18 years diagnosed with autoimmune encephalitis. Twenty-two patients underwent neuropsychological assessment.
ResultsAll but one of the patients presented cognitive impairment. Memory was the most frequently affected domain (90.91%), followed by language (50%), attention (45.45%), executive function (40.91%), praxis (40.91%), and visuospatial skills (9.09%). No association was found with disease progression time, age, sex, or education level. Results from cerebrospinal fluid analysis, electroencephalography, and brain magnetic resonance imaging studies revealed alterations in 77.27%, 57.89%, and 55% of cases, respectively.
ConclusionsMemory is the most frequently affected cognitive domain; however, other cognitive domains were also altered in our patients. Neuropsychological assessment is crucial to determine which cognitive domains are impaired in patients with autoimmune encephalitis.
El deterioro cognitivo, usualmente asociado a crisis epilépticas y psicosis, es uno de los principales síntomas de la encefalitis autoinmune. El objetivo de nuestro estudio fue describir las alteraciones cognitivas en pacientes con encefalitis autoinmune.
MétodosTrabajo descriptivo transversal. Se revisaron 39 registros de pacientes mayores de 18 años con diagnóstico de encefalitis autoinmune, 22 de los cuales tuvieron evaluación neuropsicológica.
Resultadosde los 22 pacientes sólo uno no presentó deterioro cognitivo. El dominio más comprometido fue la memoria (90,91%), seguido del lenguaje (50%), atención (45,45%), funciones ejecutivas (40,91%), apraxia (40,91%) y habilidades visuoespaciales (9,09%). No se encontró asociación con el tiempo de enfermedad, grado de instrucción, sexo o edad. Los análisis de líquido cefalorraquídeo, encefalograma y resonancia magnética estuvieron alterados en 77,27%, 57,89% y 55% de los casos respectivamente.
ConclusionesEl trastorno de memoria es el compromiso más frecuente; sin embargo, nosotros encontramos que nuestros pacientes presentaban alteraciones de otras áreas cognitivas. La evaluación neuropsicológica es fundamental para determinar qué dominios están comprometidos en los pacientes con encefalitis autoinmune.
Autoimmune encephalitis (AE) is inflammation of brain tissue that causes neurological dysfunction and is triggered by immunological factors such as antibodies or cell immunity against antigens present in the brain parenchyma.1 The most frequent form in paediatric patients is acute disseminated encephalomyelitis (ADEM); in adults, however, anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis is the leading cause of antineuronal antibody-mediated encephalitis and, according to some studies, is more frequent than any type of viral encephalitis.2 Presentation is subacute, mainly affecting young adults, predominantly women, and is associated with certain tumours.1 It typically presents with behavioural and cognitive alterations, seizures, psychotic symptoms, abnormal movements, and autonomic and respiratory dysfunction, and is included in the differential diagnosis of acute psychosis and rapidly progressive dementia.1,3 Cognitive impairment is mainly characterized by memory complaints and executive dysfunction.4,5
In 30%–90% of cases, AE causes focal alterations on brain MRI, inflammatory changes in the cerebrospinal fluid (CSF), or focal or diffuse epileptic activity in electroencephalography studies (EEG).5,6 Diagnosis of autoimmune encephalitis is classified as probable or definite according to whether autoantibodies are detected.7 Treatment is based on evidence from observational studies and expert opinion, and is classified as first-line (corticosteroids, plasmapheresis, human immunoglobulins) or second-line treatment (rituximab, cyclophosphamide). Treatment should be started as soon as probable autoimmune encephalitis is diagnosed, and must be combined with an active search for and resection of the tumour. Over 90% of patients respond to first-line therapy, with 50% improving within 4 weeks; the mortality risk is very low if the disease is detected early and treated aggressively, although rates may reach 10%.4,8
The cognitive alterations described in the literature vary according to the time of assessment, type of antibody detected, and neuropsychological tool used.8 The purpose of this study is to analyse cognitive impairment in adult patients with AE attended at a national reference centre for neurological diseases in Peru.
Patients and methodsWe conducted a descriptive, cross-sectional study of the clinical records of patients hospitalised at the Peruvian National Institute of Neurological Sciences between January 2015 and December 2019 with a diagnosis of probable or definite AE according to the criteria proposed by Graus et al.7 In order for patients to be included in our study, they had to be over 18 years of age and have completed a neuropsychological evaluation during hospitalisation. One patient was excluded due to a lack of laboratory data supporting the diagnosis.
All patients admitted to our centre with encephalitis undergo MRI, CSF analysis, EEG, and chest, abdomen, and pelvis CT (to rule out occult neoplasia), although it is not always possible to perform all complementary tests. Diagnosis of definite AE was established when the antibody was detected or when MRI findings were highly suggestive (limbic encephalitis and ADEM) and CSF analysis ruled out infection. When no antibody was detected but MRI studies and CSF and blood analyses ruled out other aetiologies, we established a diagnosis of probable AE.
Cognitive assessments were performed by neuropsychologists from our centre’s behavioural neurology department. The neuropsychological test battery included: (1) the Wechsler Adult Intelligence Scale (WAIS-III), which has previously been validated in a Chilean population and whose scores have been standardised according to age and education level9; (2) the NEUROPSI battery, created in Mexico, which evaluates orientation, attention, memory, language, visuospatial ability, executive function, and reading, writing, and mathematics10; (3) the Rey-Osterrieth Complex Figure test (copy condition only) to evaluate attention, concentration, planning, fine motor coordination, and constructional praxis11; and (4) the Rey Auditory Verbal Learning Test (RAVLT), an auditory memory test that evaluates verbal learning and immediate recall of a list of 15 words.12 We also used the Zung Self-Rating Anxiety Scale and the Zung Self-Rating Depression Scale.13 In order to compare full-scale, verbal, and performance IQ scores of the Wechsler Intelligence Scale, scores were converted to T-scores; the average score was set at 100, with scores below 70 considered to indicate intellectual disability. The NEUROPSI battery has a maximum score of 130; normative values were adjusted for age and education level. The maximum score on the Rey-Osterrieth Complex Figure is 18; scores <16 points indicate poor performance. The RAVLT has a maximum score of 15, with cut-off scores ranging from 11 to 14 depending on age and education level, according to normative data from a Chilean population.14 Patients were considered to present cognitive impairment if they performed poorly in at least one neuropsychological test.
We analysed the following variables: sex, age, education level, disease progression time, epileptic seizures, psychotic symptoms, cognitive impairment, type of encephalitis, CSF findings, EEG results, and brain MRI findings.
Data were anonymised and analysed with the Stata software, version 16. Categorical variables are expressed as frequencies and percentages. Continuous variables were tested for normality with the Shapiro–Wilk test; normally distributed variables are expressed as means and standard deviations (SDs), and non-normally distributed variables as medians and interquartile ranges.
We also explored the associations between the dependent variable (cognitive impairment) and independent variables (age, sex, disease progression time, CSF alterations) using the t-test, Pearson correlation coefficient, chi-square test, and Fisher exact test. Statistical significance was set at P < .05. This study complies with the ethical principles of the Declaration of Helsinki (2013 revision) and was approved by our centre’s research ethics committee.
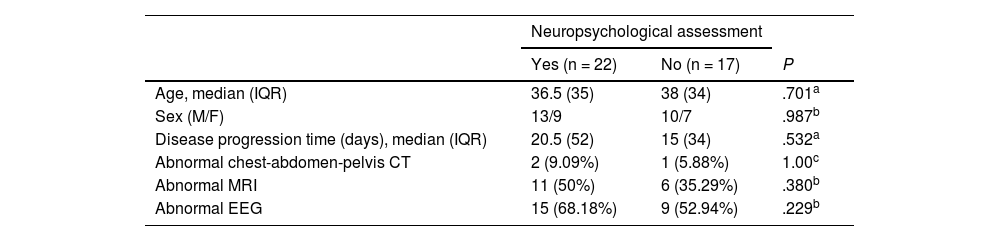
ResultsWe gathered data from 39 patients with a mean age (SD) of 39.23 (17.55) years; 58.97% (n = 23) of patients were men. Twenty-nine patients had probable AE and 10 had definite AE (5 with limbic encephalitis, 4 with anti-NMDAR encephalitis, and 1 with ADEM). Most patients underwent at least one complementary test to rule out occult neoplasia (teratoma in 1, seminoma in 1, lymphoproliferative disease in 1). Clinical data are summarised in Table 1.
Clinical characteristics of our sample of patients with autoimmune encephalitis.
| Neuropsychological assessment | |||
|---|---|---|---|
| Yes (n = 22) | No (n = 17) | P | |
| Age, median (IQR) | 36.5 (35) | 38 (34) | .701a |
| Sex (M/F) | 13/9 | 10/7 | .987b |
| Disease progression time (days), median (IQR) | 20.5 (52) | 15 (34) | .532a |
| Abnormal chest-abdomen-pelvis CT | 2 (9.09%) | 1 (5.88%) | 1.00c |
| Abnormal MRI | 11 (50%) | 6 (35.29%) | .380b |
| Abnormal EEG | 15 (68.18%) | 9 (52.94%) | .229b |
CT: computed tomography; EEG: electroencephalography; F: female; IQR: interquartile range; M: male; MRI: magnetic resonance imaging.
Only 22 patients underwent neuropsychological assessment, which was conducted 1–2 weeks after the final bolus of methylprednisolone; therefore, the following analyses refer only to this subgroup.
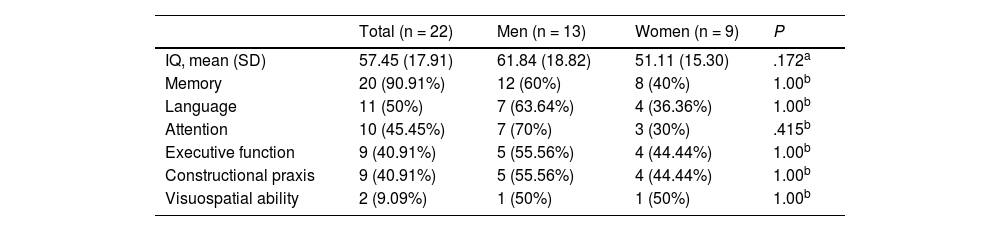
Mean age (SD) in the subgroup of patients undergoing neuropsychological assessment was 39.31 (17.20) years; 59.09% were men (13/22). By education level, 3 patients (13.64%) had completed primary education only, 9 (40.91%) had completed secondary education only, and 10 (45.45%) had higher studies. The median disease progression time at admission was 20.5 days. Epileptic seizures were reported in 81.82% of patients (18/22) and psychotic symptoms in 18.18% (4/22). Only two patients presented depression and another presented anxiety. Fifteen patients (68.18%) were diagnosed with probable AE and 7 (31.82%) with definite AE (4 with limbic encephalitis, 2 with anti-NMDAR encephalitis, and 1 with ADEM). All patients but one presented impairment of at least one cognitive domain; this was considered sufficient to establish a diagnosis of cognitive impairment. Table 2 presents the cognitive domains affected in our sample.
Cognitive domains presenting impairment in the subgroup of patients with autoimmune encephalitis who underwent neuropsychological assessment.
| Total (n = 22) | Men (n = 13) | Women (n = 9) | P | |
|---|---|---|---|---|
| IQ, mean (SD) | 57.45 (17.91) | 61.84 (18.82) | 51.11 (15.30) | .172a |
| Memory | 20 (90.91%) | 12 (60%) | 8 (40%) | 1.00b |
| Language | 11 (50%) | 7 (63.64%) | 4 (36.36%) | 1.00b |
| Attention | 10 (45.45%) | 7 (70%) | 3 (30%) | .415b |
| Executive function | 9 (40.91%) | 5 (55.56%) | 4 (44.44%) | 1.00b |
| Constructional praxis | 9 (40.91%) | 5 (55.56%) | 4 (44.44%) | 1.00b |
| Visuospatial ability | 2 (9.09%) | 1 (50%) | 1 (50%) | 1.00b |
IQ: Wechsler Adult Intelligence Scale intelligence quotient; SD: standard deviation.
The Mann–Whitney U test revealed that the median IQ was significantly higher in patients aged ≤38 years than in those older than 38 (P = .027). However, IQ scores were similar in patients with disease progression times above and below 3 weeks (P = .843). Likewise, no association was found between IQ score and education level.
The Spearman correlation coefficient showed no correlation between IQ and disease progression time (rho = –0.002; P = .991), IQ and CSF protein level (rho = –0.142; P = .527), or IQ and CSF cell count (rho = –0.065; P = .773). We only found a moderate, inverse correlation between IQ and age (rho = –0.383; P = .078), although it did not reach the threshold for statistical significance.
CSF analysis revealed alterations in 17 patients (77.27%): 8 patients presented high protein levels, 2 presented high protein levels plus pleocytosis, and 7 presented pleocytosis only. Twenty-two patients underwent neuroimaging studies, 11 of whom (50%) presented focal alterations compatible with encephalitis. EEG studies were performed in 19 patients, 11 of whom (57.89%) displayed alterations suggestive of diffuse encephalopathy or epileptiform activity.
DiscussionAutoimmune encephalitis is more frequent among women and may present between the ages of 2 and 67 years.1,15 However, below the age of 12 years, the condition predominantly affects boys.4 Interestingly, our sample included a slightly higher percentage of men; mean age was within the range reported in the literature.
Cognitive alterations appear from the early stage of AE and continue to develop as the disease progresses. The pathophysiology of cognitive impairment has been widely studied, mainly in patients with anti-NMDAR antibodies. NMDAR modulates excitatory synaptic transmission in the CNS and is essential for long-term potentiation, the neural basis of learning and memory. In encephalitis, IgG antibodies target the NR1 subunit of the receptor, causing receptor internalisation and decreasing NMDA-mediated current amplitudes.16 NMDAR is abundantly expressed in the frontal and hippocampal cortex, which explains why the disease is predominantly associated with alterations in memory and executive function.17 For a more in-depth description of the pathophysiological substrate of cognitive impairment according to the type of autoantibody involved, we recommend the study by Gibson et al.18 The course of AE begins with a prodromal phase, followed by a psychotic phase, characterised by neuropsychiatric symptoms19; in this phase, 32%–50% of patients present cognitive symptoms.20–22 In our sample, all patients but one presented cognitive impairment, whereas a study of 86 Chinese patients does not mention cognitive impairment among the clinical manifestations of AE (except for allomnesia, among the psychiatric symptoms).23 This may be explained by three factors. Firstly, differences in the time when the neuropsychological battery was administered, which is not indicated in most studies.1,19,20,22–24 Cognitive function is difficult to evaluate during the first days of hospitalisation due to agitation, sensory alterations, and recurrent seizures.1,24,25 In our sample, the psychometric assessment was performed 1–2 weeks after administration of the final bolus of methylprednisolone, despite which nearly all patients displayed cognitive alterations. Secondly, different studies use different neuropsychological test batteries, and these are not always mentioned in the articles.19,20,22,24 Our battery included several tests validated in Latin American populations,9–12 such as the WAIS-III and RAVLT; these tests were also used by Flanagan et al.5 Finally, studies differ in the type and severity of encephalitis reported: while we included patients diagnosed both with probable and with definite AE, most publications only include patients with definite AE, and describe cognitive impairment according to the type of antibody detected,1,15,22,23,25 which hinders comparison against our results.
The cognitive profile of AE is characterised mainly by memory impairment (affecting working, short-term, and anterograde memory), and also involves executive dysfunction.1,5,22,26 However, a review including patients with anti-NMDAR encephalitis found impaired concentration, apraxia, aphasia, and anterograde and retrograde amnesia.19 Another review including 383 patients with AE from Latin America reports similar results.21 These findings are consistent with our own results: in our sample, memory was the most frequently affected cognitive domain, followed by attention, language, constructional praxis, executive function, and visuospatial ability. However, studies including adolescents most frequently report language disorders, characterised by decreased verbal output progressing to mutism.25 Regarding IQ, Flanagan et al.5 report a mean score of 89 in 51 patients with autoimmune dementia; this score is higher than that observed in our sample.
Epileptic seizures are observed in up to 48% of patients with AE presenting cognitive impairment.27 In our sample, over 80% of patients presented this complication. The importance of this finding lies in its association with the development of cognitive impairment,28 particularly when the pathophysiological substrate of seizures is found in the temporal lobe; the main cognitive domains affected in these cases are memory and executive function.29 Furthermore, the percentage of patients with psychotic symptoms in our sample was considerably lower than reported in the literature, considering that these symptoms are reported in up to two-thirds of patients.1,4
The systematic review by Blinder and Lewerenz6 reports CSF pleocytosis in up to 50% of patients, with a mean count of 20 cells/μL, high CSF protein levels in up to 53% of patients, and pleocytosis plus high protein levels in 25%–100% of cases. These findings stand in contrast with our results, as two-thirds of our patients presented CSF alterations, but only two patients presented both pleocytosis and high protein levels. Furthermore, five patients presented no CSF inflammatory changes, which seems to be frequent according to the literature.30,31 Flanagan et al.5 report brain MRI alterations in 29% of patients and epileptiform activity in the EEG study in 18%, whereas Titulaer et al.4 report MRI alterations in 33% and EEG alterations in 90%. In our sample, half of the patients undergoing MRI and EEG studies displayed alterations.
The studies conducted to date with patients with AE do not describe an association between presence of cognitive impairment and such factors as age, sex, or disease progression time.1,4,5 Our study only found a correlation between lower IQ and age above 38 years; this is not surprising, however, since age is a recognised risk factor for cognitive impairment in other diseases.32 We found no significant correlations with the remaining factors analysed; future prospective studies with larger samples may provide valuable information on this topic.
Our study has several limitations, including the small sample size and the fact that patients were selected by convenience sampling, which prevents us from extrapolating our results or identifying associated factors. The design of our study prevents us from providing a detailed description of the progression of cognitive impairment. Furthermore, antibody studies were not performed for all patients; therefore, the percentage of patients with definite AE may be underestimated. Neuropsychological test performance may be influenced by treatment with methylprednisolone. In any case, this is the first study of cognitive impairment in a representative sample of patients with AE from a national-level reference centre in Peru. Few studies have provided detailed descriptions of the neuropsychological tools used to evaluate cognitive performance in the acute phase of AE.1,5 Neuropsychological assessment during the acute phase represents a major challenge due to the severity of the associated symptoms; a qualitative description of cognitive function is frequently performed in this stage, with standardised tests being more feasible in the subacute and chronic phases of AE.18
In conclusion, our study shows that AE affects not only memory but also executive function, language, attention, and praxis. Therefore, a comprehensive neuropsychological assessment of these patients, including tests for all cognitive domains, should be performed starting in the acute phase, and repeated periodically. Future studies should aim to describe the peculiarities of cognitive impairment in each type of AE, explore associated factors, and measure treatment response.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.