Emerging research has highlighted COVID-19's profound impact on the nervous system, with ongoing debate regarding its severity and the emergence of peripheral neurological disorders. This study delves into the correlation between COVID-19 severity and electromyographic abnormalities in hospitalized patients, aiming to elucidate the nature and extent of neuromuscular dysfunction associated with the disease.
MethodsIn a retrospective, cross-sectional, observational study, we analyzed data from 170 patients treated at the North Physical Medicine and Rehabilitation Unit between July 2020 and April 2021. Post-COVID-19 patients underwent comprehensive motor and sensory nerve conduction studies. Spearman's correlation and Kruskal–Wallis tests were used to examine the relationship between disease severity and electromyographic findings.
ResultsOf the 170 patients in the study, 71.17% were male and 28.82% female, with an average age of 48 years; the subgroup of patients with severe clinical classification predominated at 68.23%. Spearman's correlation yielded values higher than 0.5 for all parameters analyzed concerning severity classification. A significant difference was found in the average of non-evoked motor and sensory nerves, motor nerves with latency, amplitude, and conduction velocity impairment, and affected muscles regarding critical classification.
ConclusionOur study showed a relationship between the severity of COVID-19 and an increase in non-evoked motor and sensory nerves, impaired motor nerve latency, amplitude, and velocity, and more affected muscles, particularly in critical cases.
Investigaciones emergentes han destacado el profundo impacto del COVID-19 en el sistema nervioso, con un debate en curso sobre su gravedad y la aparición de trastornos neurológicos periféricos. Este estudio profundiza en la correlación entre la gravedad del COVID-19 y las anomalías electroneuromiográficas en pacientes hospitalizados, con el objetivo de dilucidar la naturaleza y el alcance de la disfunción neuromuscular asociada con la enfermedad.
MétodosEn un estudio retrospectivo, transversal y observacional, analizamos datos de 170 pacientes atendidos en la Unidad de Medicina Física y Rehabilitación del Norte entre julio de 2020 y abril de 2021. Los pacientes posteriores a COVID-19 se sometieron a estudios completos de conducción nerviosa motora y sensorial. Las pruebas de correlación de Spearman y Kruskal-Wallis se utilizaron para examinar la relación entre la gravedad de la enfermedad y los hallazgos electroneuromiográficos.
ResultadosDe los 170 pacientes del estudio, 71.17% correspondieron al sexo masculino y 28.82% al femenino; con edad promedio de 48 años; predominando el subgrupo de pacientes con clasificación clínica grave con el 68.23%. Respecto a la correlación de Spearman se obtuvieron valores mayores a 0.5 en todos los parámetros analizados respecto a clasificación de severidad. Se encontró diferencia significativa en promedio de nervios motores y sensoriales no evocados, nervios motores con afectación en latencia, amplitud y velocidad de neuroconducción, y de músculos afectados respecto a clasificación crítica.
ConclusionesNuestro estudio mostró una relación entre la gravedad de la COVID-19 y el aumento de los nervios motores y sensoriales no evocados, el deterioro de la latencia, amplitud y velocidad de los nervios motores, y más músculos afectados, especialmente en casos críticos. Sin embargo, no se encuentra correlación directa entre la gravedad de la COVID-19 y los hallazgos electroneuromiográficos en los pacientes evaluados.
In December 2019, numerous cases of pneumonia-like illness with an unknown etiology were reported in China's Hubei region within Wuhan Province. By January 2020, this illness was identified as a novel type of coronavirus (an RNA virus now recognized as SARS-CoV-2) that had not been previously identified in humans. The disease caused by this virus is known as COVID-19 (an acronym derived from “coronavirus disease” in English and “19” referring to the year of its initial identification). The World Health Organization (WHO) officially declared it a pandemic in March 2020.1,2
Among the nervous system disorders associated with COVID-19, several have been extensively reported, including cerebrovascular accidents such as cerebral venous sinus thrombosis, ataxia, seizures, a depressed level of consciousness, encephalopathy, acute polyneuropathy, critically ill polyneuropathy, encephalitis, epilepsy, and myopathy. Additionally, various symptoms such as headache, myalgia, fatigue, hypogeusia, hyposmia, and anosmia are more frequently observed in individuals with severe illness, medical comorbidities, and other vascular risk factors.3–6
The COVID-19 pandemic has surpassed respiratory illnesses, leading to a significant occurrence of neuropathies and other neurological aftereffects. Consequently, the prevalence of neurological complications necessitates an extensive evaluation beyond respiratory symptoms, underscoring the complex influence of SARS-CoV-2 on the nervous system.7
Particularly in ICU critical patients, the precise diagnosis of neuropathies is crucial for tailoring therapeutic and rehabilitative strategies aimed at markedly enhancing prognoses and post-infection life quality. This viewpoint stresses the need to identify adjustable factors, both pharmacological and non-pharmacological, to alleviate the long-term impacts of these neurological issues, positioning it at the forefront of innovation in neurology and rehabilitation medicine.8,9
Therefore, our attention to peripheral nerve injuries in COVID-19 patients has, to date, pinpointed axonotmesis damage as the central associated injury. The following concurrent mechanisms are mentioned:
- •
Post-infectious inflammatory neuropathy: Peripheral nerve injuries stemming from post-infectious inflammation occur because of immune-mediated mechanisms. Guillain-Barré syndrome, particularly its acute inflammatory demyelinating polyneuropathy subtype, has been frequently documented in multiple case reports.1,10,11
- •
Hospitalization sequelae: Stretch or compression injuries related to prone positioning or distal symmetric polyneuropathy.
- •
Distal symmetrical polyneuropathy: This is the most common type of peripheral neuropathy, which can be associated with a wide variety of causes, including diabetes, nutritional deficiencies, toxic etiologies, infections, or inflammatory conditions. It has also been observed that critical patient polyneuropathy is a distal symmetrical polyneuropathy linked to prolonged hospital stays in the intensive care unit.12
- •
Nerve entrapment due to occupant injury: This occurs due to the focal compression of a peripheral nerve due to adjacent injury, such as hematoma, tumor, or bony structures, or within an anatomical tunnel. This condition could arise in COVID-19 patients due to anticoagulant use and the development of hematomas or accumulations of drainable fluids. Several case reports highlight a correlation between another type of peripheral nerve injury, mono neuritis multiplex, and SARS-CoV-2 infection.13
Additionally, the severity of COVID-19 is classified according to its degree, for which the criteria established by the WHO are used, categorizing the disease as mild, moderate, severe, and critical.14 This classification is given by:
Mild:
- 1.
Symptoms of COVID-19 without pneumonia.
- 2.
Oxygen saturation (SpO2) ≥94% on room air.
- 3.
Normal respiratory rate (≤22 breaths per minute).
Moderate:
- 1.
Presence of pneumonia.
- 2.
SpO2 ≥90% on room air.
- 3.
Normal respiratory rate (≤22 breaths per minute).
- 4.
No signs of dyspnea at rest.
Severe:
- 1.
SpO2 <90% on room air.
- 2.
Respiratory rate >22 breaths per minute.
- 3.
Dyspnea at rest.
- 4.
Presence of tachypnea, use of accessory respiratory muscles, intercostal retractions, and/or cyanosis.
Critical:
- 1.
Respiratory failure requiring invasive mechanical ventilation.
- 2.
Septic shock.
- 3.
Multiorgan failure.
On the other hand, the increased severity of COVID-19 correlates with a higher prevalence and severity of peripheral neuropathies and movement disorders. This underscores the significant long-term neurological impact of the disease. Our preliminary results indicate an association between critical COVID-19 cases and greater neuromuscular involvement, affecting both motor and sensory nerves. This study highlights the importance of further investigating post-COVID-19 neuromuscular sequelae and incorporating electrophysiological studies into patient management for assessing and managing neuromuscular disabilities. This will improve prognosis and facilitate rehabilitation. Our primary objective is to clarify the relationship between COVID-19 severity and electrophysiological findings in hospitalized patients. This will provide insights into the long-term neurological implications of the disease and guide personalized care strategies.
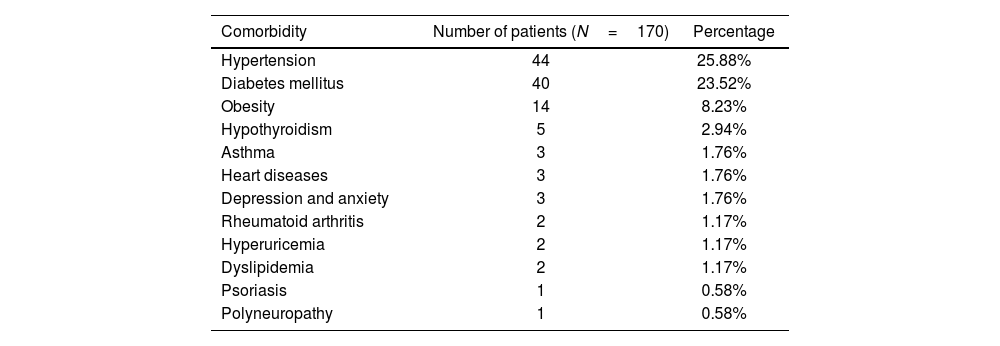
Material and methodsStudy designA comprehensive review was conducted utilizing clinical records to gather the frequencies of various comorbidities (Table 1). Additionally, an examination of electroneuromyographic reports from patients at the electrodiagnostic service was undertaken.
Prevalence of comorbidities in COVID-19 patients. It presents both the number and percentage of patients with each comorbidity, based on a total sample size of 170 patients.
| Comorbidity | Number of patients (N=170) | Percentage |
|---|---|---|
| Hypertension | 44 | 25.88% |
| Diabetes mellitus | 40 | 23.52% |
| Obesity | 14 | 8.23% |
| Hypothyroidism | 5 | 2.94% |
| Asthma | 3 | 1.76% |
| Heart diseases | 3 | 1.76% |
| Depression and anxiety | 3 | 1.76% |
| Rheumatoid arthritis | 2 | 1.17% |
| Hyperuricemia | 2 | 1.17% |
| Dyslipidemia | 2 | 1.17% |
| Psoriasis | 1 | 0.58% |
| Polyneuropathy | 1 | 0.58% |
The severity of COVID-19 was classified using the criteria established by the WHO and the “Clinical Guide for the Treatment of COVID-19 in Mexico”. This classification allowed for accurate diagnosis and treatment, as well as precise comparison between patients.14,15
Additionally, following the guidelines of the “Clinical Guide for the Treatment of COVID-19 in Mexico,” a distinction was made between mild to moderate COVID-19 cases, which do not require hospitalization, and severe to critical cases, which need intensive care.15 This involved assessing respiratory function, the need for ventilatory support, and the presence of complications such as severe pneumonia. This classification allowed for the analysis of the correlation between the severity of COVID-19 and findings in electromyographic studies.
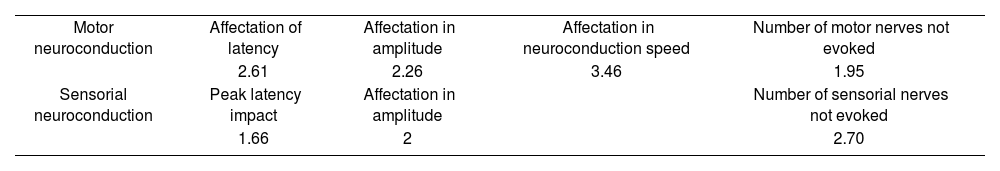
Neurological assessment protocolThe adopted approach protocol encompassed neuroconduction studies of the thoracic and pelvic limbs. These studies included assessments of the median, ulnar, and radial nerves for both motor and sensory functions; peroneal and tibial nerves for motor functions; and superficial peroneal and sural cutaneous nerves for sensory functions. Sensory neuroconduction tests were evaluated using an antidromic technique, while motor neuroconductions were assessed using an orthodromic technique. Furthermore, specific muscles underwent studies using monopolar needle electrodes (setup/placement adhering to the technique outlined by Preston), as outlined in Table 2. During electromyography, analyses were performed under various conditions: rest (insertion, spontaneous activity), minimal activity (motor unit action potential analysis), moderate activity, and maximal activity (recruitment and interference pattern assessment). The utilized electromyography equipment was the Natus Nicolet Viking Quest software.
Abnormal results in electroneuromyography studies (N=170).
| Motor neuroconduction | Affectation of latency | Affectation in amplitude | Affectation in neuroconduction speed | Number of motor nerves not evoked |
| 2.61 | 2.26 | 3.46 | 1.95 | |
| Sensorial neuroconduction | Peak latency impact | Affectation in amplitude | Number of sensorial nerves not evoked | |
| 1.66 | 2 | 2.70 |
The selection process involved patients with a COVID-19 history who satisfied the inclusion criteria. Regarding inclusion criteria, eligible patients attended and had undergone electroneuromyographic studies and comprehensive clinical records. Specifically, patients in an outpatient state beyond the acute phase were chosen, and the normality parameter was determined according to Preston's criteria. Exclusion criteria comprised patients needing electroneuromyographic studies in their records, incomplete study protocols, or insufficient information.
Statistical analysisStatistical analysis was conducted using R IDE Studio software, version 4.2. To assess data normality, the Kolmogorov–Smirnov Lilliefors test was employed. However, a p-value of <.05 emerged, signifying a departure from a normal distribution. Correspondingly, Levene's test for homoscedasticity also yielded a p-value of <.05, implying variance inequality among groups. Given the contravention of normality and homogeneity assumptions, the decision was made to employ the Spearman correlation coefficient for variable relationship analysis and the Kruskal–Wallis test for inferential analysis. We do not show the Spearman correlation results since this was not statistically significant (p-value >.05).
ResultsIn this study, we evaluated a group of 193 patients, of whom 23 were excluded due to non-adherence to the inclusion criteria. Consequently, our analysis focused on a final group of 170 patients, comprising 71% males, aged 25–80 years with a mean age of 48 years.
The analysis of comorbid conditions revealed hypertension and diabetes mellitus as the predominant health issues. Notably, the co-occurrence of diabetes mellitus and systemic arterial hypertension was the most common comorbidity pairing, affecting 18 participants (10.58%). A history of smoking was identified in 7 patients (4.11%).
Clinical classifications of COVID-19 within our group showed a distribution: mild symptoms in 5.88%, moderate symptoms in 8.23%, severe symptoms in 68.23%, and critical conditions in 17.64%.
Electroneurography assessments indicated abnormalities in 64.11% of the patients, highlighting diverse effects on various nerve conduction parameters. On average, muscles exhibiting membrane instability were found in 3.45 affected muscles per patient. Notably, in over 85% of the patients, the electromyographic study was conducted 3 months after the acute phase, underscoring the persistence of neuromuscular symptoms. Preliminary correlation analysis focused on motor neuron conduction values, including latency, amplitude, and conduction velocity. Detailed findings from the Kruskal–Wallis test, followed by the Wilcoxon post-hoc test where applicable, are presented subsequently.
This enhanced section integrates the requested additional detail, emphasizing the timing of electromyographic studies in relation to the acute phase of COVID-19.
Clinical classification and motor nerve conduction findingsA p-value <.05 indicated significant results regarding the classification and findings of motor nerves. This pertained to both the number of affected nerves in latency [p=0.0001153], amplitude [p=0.0004219], motor conduction velocity [p=0.001031], and non-evoked [5.36e-06]. Among these, the critical patient group observed a higher average number of affected nerves. Post-hoc testing further revealed significant differences in the number of motor nerves affected by latency: mild-critical (p=.0019) and severe-critical (p=.0019).
Distinct significant differences emerged through post-hoc testing in terms of the number of motor nerves affected in amplitude, specifically between the moderate-severe (p=.042) and severe-critical (p=.001) groups (Fig. 1). A comparable outcome arose for the number of motor nerves affected in motor conduction velocity, with a significant difference noted in the severe-critical group (p=.00085) (Fig. 1).
(A) Relationship of clinical classification of COVID-19 with the number of motor nerves affected in latency (N=170). (B) Relationship of clinical classification of COVID-19 with the number of motor nerves affected in amplitude (N=170). (C) Relationship of clinical classification of COVID-19 with the number of motor nerves affected in neuron conduction velocity (N=170). (D) Relationship of clinical classification of COVID-19 with the number of motor nerves not evoked (N=170).
Similar significant differences manifested in the number of non-evoked motor nerves across clinical classification groups, particularly within the severe-critical group (p=7.2e-06) (see Fig. 2). For non-evoked sensory nerves, only a p-value of <.05 was observed, with critical patients displaying a higher average (Fig. 1).
(E) Relationship of clinical classification of COVID-19 with the number of sensory nerves with latency involvement (N=170). (F) Relation between the clinical classification of COVID-19 with the number of sensorial nerves and affectation in their amplitude (N=170). (G) Relationship of clinical classification of COVID-19 with the number of sensory nerves not evoked (N=170). (H) More significant increase is observed in the critical stage of affected muscles, according to the myography study (N=170).
A significant distinction was noted in post-hoc testing for the number of non-evoked sensory nerves among different clinical classification groups: mild-critical (p=.0068), moderate-critical (p=.0076), and severe-critical (p=2.6e-05) (Fig. 2).
A p-value <.05 underscored significant outcomes about the number of affected muscles, predominantly among critical patients. Post-hoc testing further corroborated this significant difference in the number of affected muscles within the severe-critical group (p=2.8e-06) (Fig. 2).
DiscussionThe results obtained from this study reveal that there is no discernible correlation between the severity of COVID-19 and the findings observed in electroneuromyographic studies; this is because the statistic performed with the Spearman test was not significant.
Among the studied subgroups (Figs. 1 and 2), a notable distinction emerges between the outcomes of critically ill patients and those in non-critical conditions. This difference is particularly pronounced in the results of motor nerves, where the degree of impairment was more substantial. Notably, within the context of sensory neuron conduction studies and across distinct clinical classification groups, the sole significant difference lay in the count of non-evoked sensory nerves, which pertained predominantly to the critical classification in contrast to other severity types.
A significant distinction persisted within the severe and critical patient groups regarding myography studies.
While no prior research endeavors have explicitly aimed to establish a direct correlation between COVID-19 severity and involvement in electromyography studies, our findings align with the existing body of knowledge. These aligning data, coupled with the heightened disease severity and the concurrent alterations in neuron conduction studies and myography, are consistent with previous research by Frithiof et al.6 This earlier study highlights a heightened prevalence of myopathies and neuropathies in individuals with a history of ICU stay and severe SARS-CoV-2 infection.
In our analysis, the differentiation in neuromuscular involvement between critical patients and those of lesser severity reflects the complexity of neuromuscular complications in COVID-19, aligning with literature that links disease severity to a higher prevalence of neuropathies and myopathies.16,17 Furthermore, we observed that comorbidities such as hypertension and diabetes mellitus could influence the severity of electromyographic alterations since diabetes is associated with an increased risk of peripheral neuropathies, which could exacerbate neurological complications in COVID-19 patients.18 Similarly, hypertension could be related to vascular changes affecting neuromuscular function.19
The presence of peripheral neuropathies in post-COVID patients suggests that pre-existing comorbidities may worsen neuromuscular damage, indicating a multifactorial mechanism in the pathogenesis of electromyographic alterations.20 Integrating these findings could enhance the management of post-COVID-19 patients, highlighting the importance of a personalized approach that considers the severity of the infection and underlying comorbidities. Therefore, incorporating these findings into clinical practice could improve the management and rehabilitation of post-COVID-19 patients, emphasizing the need for a personalized approach that considers both the severity of the infection and the underlying comorbidities.16
Additionally, our findings are in harmony with the observations of Tang et al., 21 who identify diabetes mellitus and hypertension as the most prevalent comorbidities among infected patients. However, there is a discrepancy in the reported average age; while these authors report a mean age of 59.4 years, our study subjects exhibited an average age of 43 years. Furthermore, regarding clinical classification, our data diverge from the WHO records, which document a higher incidence of mild and moderate cases.
On the other hand, critical illness polyneuropathy (CIP) is a complication observed in ICU patients with COVID-19. These findings include sensory-motor polyneuropathy with an axonal neurogenic pattern, which could have significant implications for these patients' functional recovery and rehabilitation strategies. This underscores the need to consider CIP when assessing and planning treatment for the recovery of critical COVID-19 patients.22
Continuing with this, peripheral neuropathy in COVID-19 patients is common and predominantly due to immune mechanisms or neurotoxic side effects of the medications used to treat COVID-19 symptoms. Additionally, compression of peripheral nerves due to prolonged immobilization in the ICU and preexisting risk factors such as diabetes also contribute to this condition. Therefore, it is essential to cautiously administer neurotoxic drugs such as daptomycin, linezolid, lopinavir, ritonavir, hydroxychloroquine, cisatracurium, clindamycin, and glucocorticoids, ensuring proper follow-up and treatment of ICU patients to prevent SARS-CoV-2 associated neuropathy.23
These associations are undoubtedly multifaceted and not exclusively attributed to COVID-19. In a context where factors such as ICU admissions, mechanical ventilation, corticosteroid utilization, sedation, neuromuscular blockers, multiorgan failure, muscular inactivity, positioning, and specific antibiotics operate either individually, concomitantly, or synergistically, the convergence of nerve injuries due to ischemia, cytopathic hypoxia, and energy depletion becomes more pronounced.24–28
On the other hand, in most patients, electroneuromyographic alterations were presented more than 3 months after the acute phase of COVID-19, which reflects the persistence of long-term neuromuscular and cognitive symptoms, consistent with the post-COVID sequelae observed in other studies. It has seen an increase in the risk and burden of adverse neurological outcomes, such as peripheral neuropathies and movement disorders, up to 12 months after COVID-19 infection, suggesting a significant impact of the disease on long-term neurological function.7 Additionally, the research extends these findings by examining post-COVID sequelae up to 2 years after infection, highlighting that certain neurological complications, such as fatigue and musculoskeletal disorders, remain elevated compared to non-infected individuals. They underscore the importance of integrating comprehensive follow-up and tailored intervention strategies for post-COVID-19 patients.29
It is important to note that other patients also met the severe and critical classification criteria in contrast to the number of patients categorized as mild or moderate. This is an anticipated outcome, given that the reference unit for this study is a tertiary hospital, typically receiving referrals from patients with neurological, musculoskeletal, and cardiopulmonary sequelae.
While a direct correlation between COVID-19 severity and findings in electroneuromyographic studies eludes our investigation, our results indicate a clear association between the critical patient condition and heightened neuromuscular involvement, spanning motor and sensory nerves. These findings underscore the significance of incorporating electrophysiological studies into the comprehensive management of post-COVID-19 patients. A focal emphasis on assessing and managing neuromuscular impairments is essential for enhancing prognosis and facilitating rehabilitation. Further research is warranted to grasp these observations' underlying mechanisms and clinical implications fully. This pursuit will undoubtedly advance studies' understanding, treatment, and prognosis as the intricate physiopathological spectrum intertwined with SARS-CoV-2 infection unfolds.
ConclusionOur study demonstrates a significant association between severe COVID-19 cases and various neuromuscular impairments. These include non-evoked motor and sensory nerve abnormalities and impairments in motor nerve latency, amplitude, and conduction velocity. Critical patients exhibited the most significant neuromuscular involvement. While not all patients displayed a direct correlation between disease severity and electroneuromyographic findings, our results highlight the complex and potentially multifactorial nature of COVID-19's impact on neuromuscular health. Further investigation is crucial to understand these complexities and develop personalized rehabilitation strategies for affected patients. Our work underscores the importance of neuromuscular assessments for comprehensive care of COVID-19 patients, especially those with severe presentations.
Ethical considerationsProtection of Individuals and AnimalsThe authors state that no experiments involving humans or animals were conducted for this research.
Confidentiality of dataThe authors confirm that this article does not include patient data
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNone.
Contributed investigation, software, methodology.
Contributed investigation, resources, writing, visualization.