Pulmonary calcifications are typically incidental asymptomatic findings detected through chest radiography or computed tomography scans that can be easily overlooked, with their significance often underestimated, particularly in an apparently asymptomatic individual.1 Diffuse pulmonary calcifications are caused mainly by two mechanisms: metastatic and dystrophic. Metastatic calcification involves the deposition of calcium on healthy lung tissue, while dystrophic calcification occurs on damaged lung tissue.2
Metastatic lung calcification is a metabolic disorder characterized by calcium deposition in lung parenchyma secondary to benign or malignant diseases that directly or indirectly cause increased serum calcium levels.3 Benign causes include chronic renal failure, primary or secondary hyperparathyroidism, exogenous administration of calcium and vitamin D, sarcoidosis, osteoporosis, and osteitis deformans. The most frequent malignant etiologies include massive osteolysis due to metastasis, multiple myeloma, parathyroid carcinoma, leukemia, lymphoma and breast carcinoma.4 The radiological alterations of this condition can be extremely varied. In computed tomography (CT), the most typical alterations include centrilobular nodules in the upper lobes with or without calcification, ground glass attenuation and, less frequently, dense consolidations.5
Dystrophic calcifications occur in a damaged lung following an inflammatory process, such as infection, bleeding, or pulmonary infarction. This process is local and organized, involving the deposition of crystalline hydroxyapatite calcium salt in the affected area. By definition, the serum levels of calcium and phosphate remain normal. The most common causes include healed primary granulomatous lesions (Ghon focus), characterized by a densely calcified focus situated anywhere in the lung, often in the upper lung fields, and benign tumors like hamartoma.6
We present the case of a 70-year-old male with no significant medical history, who was assessed in the emergency department for abdominal pain and a two-month progression of constitutional syndrome. A study was carried out with a blood test that highlighted complete blood count within normal range, creatinine 1.18mg/dl (CKD-EPI glomerular filtration rate 62ml/min/1.73m2), GOT 64UI/l, GPT 42UI l, LDH 1072IU/l and C-reactive protein 3.58mg/dl (normal value <0.5). Thoracoabdominal CT revealed a voluminous 14cm infiltrative mass in the upper abdomen, closely related to the lesser gastric curvature and pancreatic head. Additionally, indications of extensive peritoneal carcinomatosis surfaced, featuring loculated ascitic fluid, diffuse solid components in the omentum and pelvis, and space-occupying lesions. A lytic lesion in the left ischiopubic branch further suggested secondary involvement.
Given these findings, the study was completed with a gastroscopy that revealed a gastric mucosa with diffuse erythematous patching predominantly in the antrum and a gastric ulcer in the fundus (Forrest III). Multiple biopsies were taken, with a result of Burkitt's lymphoma (stage IV-B). Patient is starting treatment with rituximab+methotrexate+dexamethasone+cyclophosphamide+vincristine+doxorubicin.
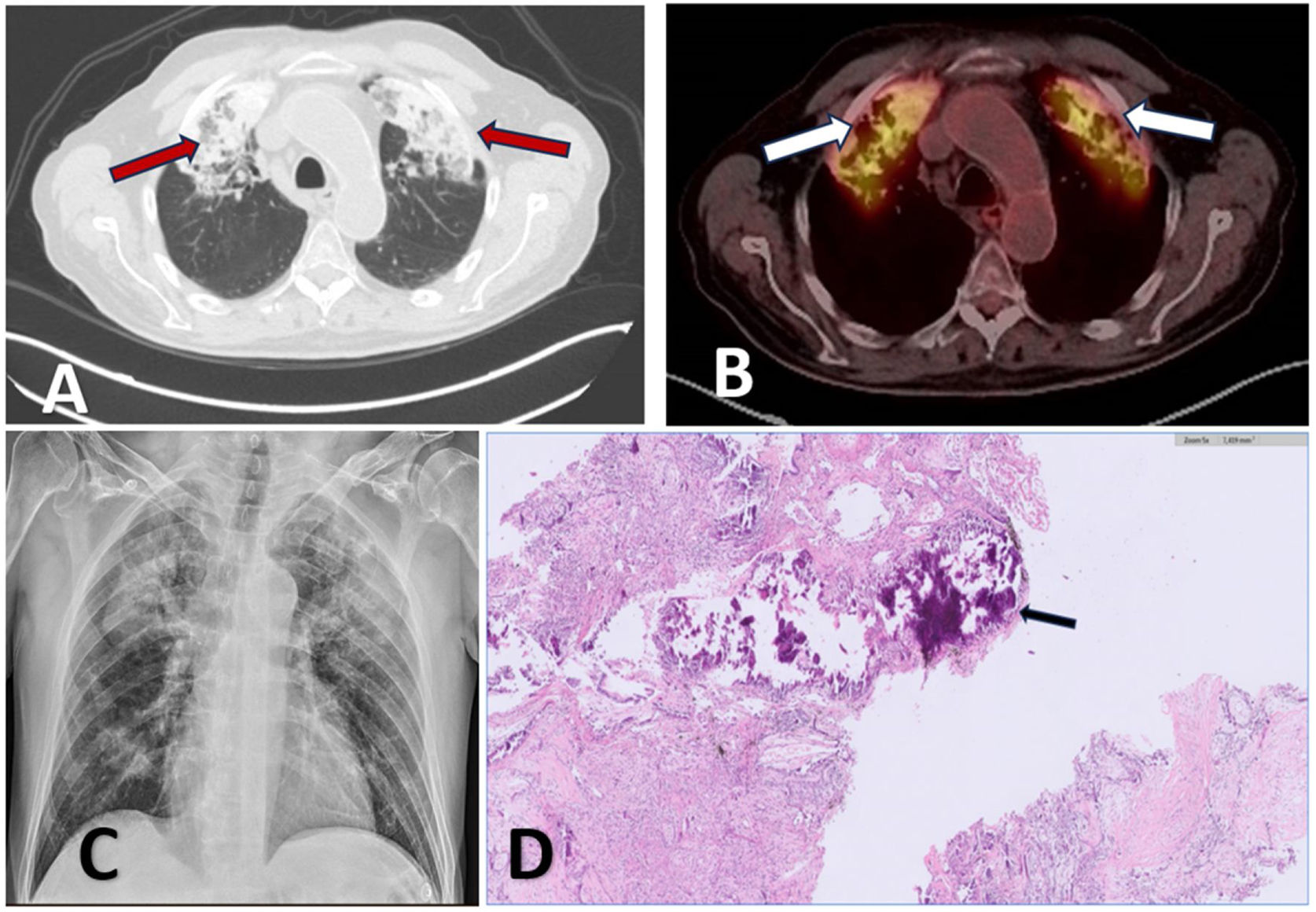
One month after completing oncohaematological treatment, and after having achieved complete remission, a chest CT (Siemens SOMATOM Definition AS 128) was performed, revealing extensive, highly attenuated consolidations with foci of calcification in the apical and anterior segments of both upper lobes with associated fibrotic changes and volume loss (Fig. 1A). The study was completed with a control thoracoabdominal positron emission tomography/computed tomography (PET-CT) (Siemens Biograph mCT), which revealed fibro-interstitial changes in both anterior segments of the upper lobes, with intense uptake of fluorodeoxyglucose (FDG) (SUVmax 7.0) (Fig. 1B). Due to these changes, as well as the dyspnea that the patient presented after moderate exertion, a diagnostic bronchoscopy was performed. No endobronchial lesions were observed. The results of the bronchoalveolar lavage are as follows: from a microbiological point of view, no microorganisms are isolated, nor are acid-fast bacilli observed; the immunophenotype is distributed in 31% neutrophils, 53% macrophages, 14% lymphocytes, CD4 47%, CD8 52%, CD4/CD8 0.90; and cytology is negative for malignancy.
(A) CT: high attenuation consolidations with foci of calcification in the apical and anterior segments of both upper lobes (red arrows). (B) PET-CT: fibro-interstitial changes in both anterior segments of the upper lobes with intense fluorodeoxyglucose uptake (right SUVmax 7.0 and left SUVmax 6.8) (white arrows). (C) Chest radiography: extensive consolidations in apical segments of both upper lobes. (D) Pathology: extensive tissue calcification, associated with fibrosis and accompanied by chronic inflammatory infiltrate with foreign body-type gigantocellular reaction (black arrow).
At this time, chronology and exposure suggest the main likelihood of toxicity is attributable to methotrexate and, more rarely, infection as an adverse effect of rituximab. Complete respiratory function tests, a 6-minute walk test, and a transthoracic echocardiogram were performed with no pathological findings. To clarify the definitive diagnosis of pulmonary consolidations, a lung cryobiopsy was performed, obtaining two samples from the anterior segment of the left upper lobe. A few days later, the pathological anatomy result was received, highlighting two fragments that measured a combined 0.8cm×0.3cm. Lung tissue is described with extensive calcification and fibrosis changes accompanied by reactive changes of the alveolar epithelium and without evidence of histological malignancy (Fig. 1D).
Serum calcium levels were a diagnostic difficulty raised in this case. Analytically, renal function and serum calcium remained within normal limits (related to the fact that before diagnosis the patient had unstudied hypocalcemia with serum calcium values of 7.7mg/dl, increasing its value to 9.5mg/dl upon diagnosis of this entity). High numbers of phosphorus were also observed, which could be responsible for lung lesions by tumor cytolysis.
The presented case is among the few described in the literature where transbronchial cryobiopsy7 was chosen as a minimally invasive diagnostic method to assist in the intriguing challenge of diagnosis, focused on the coexistence of pulmonary calcification with fibrotic changes.
The breadth of the differential diagnosis becomes a key element for precise and effective clinical management, as the hypothesis of metastatic pulmonary calcification gains relevance in the context of the patient's history of Burkitt's lymphoma. The chemotherapeutic agents used in the treatment may have contributed to the pulmonary involvement, given their association with interstitial pneumonitis and pulmonary fibrosis. It is plausible to consider the possibility of dystrophic calcification in the context of previous pulmonary alterations induced by the employed chemotherapeutic agents. This entity is commonly associated with damaged lung tissue, often a result of inflammatory processes. In this case, exposure to chemotherapeutic agents could have contributed to inflammatory changes and fibrosis in the lung tissue, providing a conducive substrate for dystrophic calcification.
In summary, this clinical case highlights the intricate nature of pulmonary calcifications and underscores the need for a comprehensive approach. Despite diagnostic uncertainty, clinical evolution remains a crucial indicator guiding our path forward.
Informed consentInformed consent was obtained from the patient for the publication of his clinical data and the use of diagnostic images.
FundingNo funding.
Authors’ contributionsM. Carrasco Sánchez, contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. E. Solís García, conceived of the presented idea and supervised the work. B.A. Paz Fernández, performed the anatomopathological study. M. García-Salmones Martín and E. Llopis Pastor, performed bronchoscopy and transthoracic lung biopsy.
Conflicts of interestThe authors have no conflict of interest to declare.