Flecainide is a class Ic antiarrhythmic used in the symptomatic control of supraventricular and ventricular cardiac arrhythmias, especially in atrial fibrillation, which is the most common tachyarrhythmia.1 Different extracardiac complications have been described due to the use of flecainide.2 Regarding lung disease, the development of diffuse interstitial involvement (pneumonitis) has been described. This condition is rare and only eight cases have been published in the literature since its inception in 1983.3–5 We present a case of a patient with flecainide-related pneumonitis, who considering the current global pandemic situation, required a screening for SARS-CoV-2 involvement within the differential diagnosis.
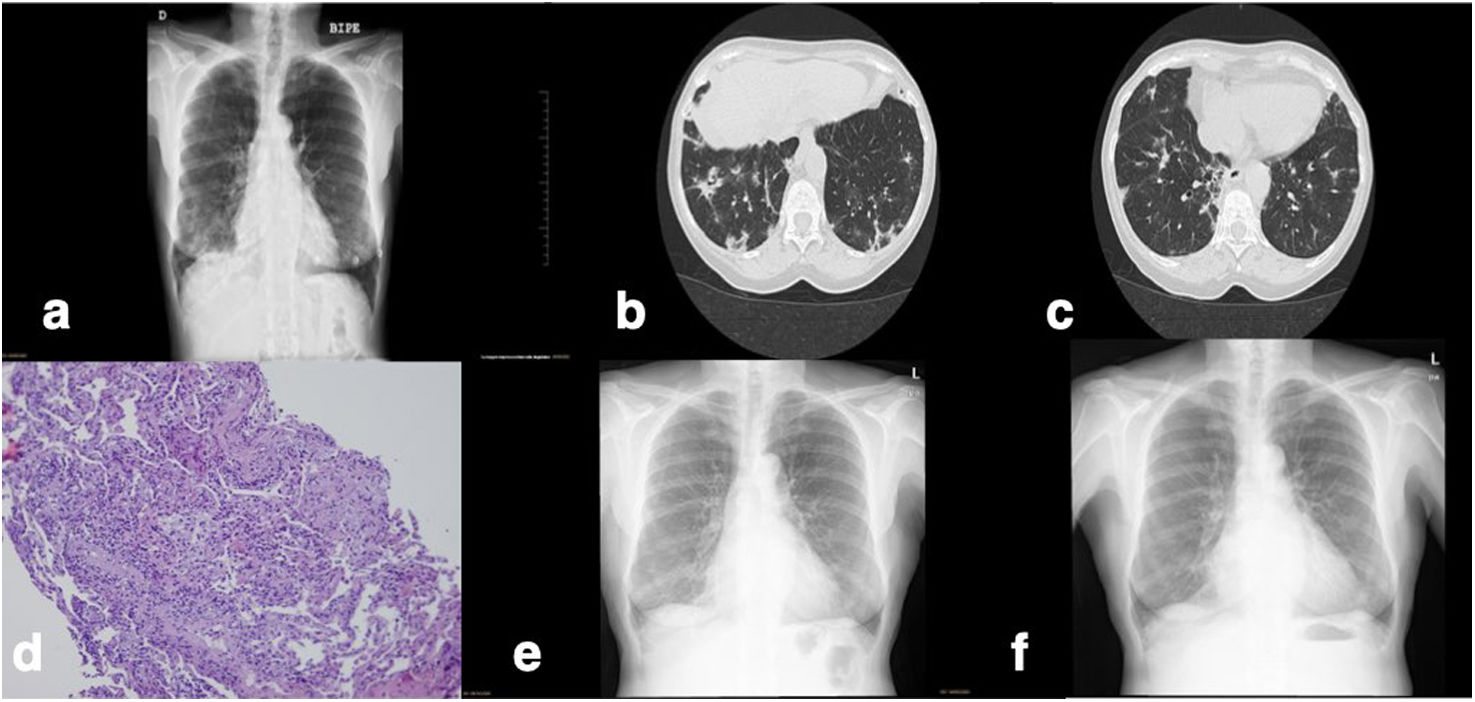
A 74-year-old woman was admitted due to one month of irritative cough and dyspnea on exertion (mMRC 1 point). She denied fever or contact with SARS-CoV-2-positive individuals. She suffered from chronic atrial fibrillation, due to poor rhythm control and the presence of palpitations, she was treated with flecainide 100mg every 24h for 2 months. An elevation of the glomerular sedimentation rate of 95mm/h was found on the blood tests. On the chest X-ray patchy opacities in both lung bases were observed. The pandemic context made it necessary to rule out COVID-19 infection (Fig. 1a). In the initial study, two nasopharyngeal swabs with polymerase chain reaction (PCR) were performed for SARS-CoV-2 as well as for other respiratory viruses such as Influenza A, B, and respiratory syncytial virus (RSV). The result was negative. SARS-CoV-2 serologies were also performed and were both IgM and IgG negative. The microbiological study was completed with atypical pneumonia serologies (IgM and IgG from Coxiella burnetii and Mycoplasma pneumoniae) that were negative. Chest computed tomography (CT) was requested for a more thorough evaluation of the infiltrates, observing multiple pseudonodular ground glass areas with an apic-basal gradient. Multiple condensations of spiculated morphology with associated small traction bronchiectasis, predominantly on the right lung, were observed in both lower lobes (Fig. 1b).
(a) The first chest X-ray upon admission of the patient. (b and c) An image of the lower lobes on CT during admission. (d) Transbronchial biopsy showing interstitial lymphocytic inflammatory infiltrate with eosinophils and pneumocyte hyperplasia. (e) Chest X-ray 4 weeks after treatment withdrawal with minimal infiltrates in the right lower lobe. (f) Chest X-ray 3 months after withdrawal of flecainide and corticosteroid treatment. It does not present infiltrates.
The patient remained afebrile, so considering the infectious etiology unlikely, the differential diagnosis was expanded. Although the patient did not present with symptoms related to connective tissue disease, an immunological study was requested with rheumatoid factor (RF), antinuclear antibodies (ANA), anti-citrullinated cyclic peptides (APCC) and complement (C3, C4) antibodies, all of which were negative. Pulmonary flecainide toxicity was one of the diagnostic possibilities that, although infrequent, was temporarily related. The orange score was calculated to assess the probability for flecainide adverse effect, the result was 5 points, which indicated that it was probable.
Finally, a bronchofibroscopy was performed in order to expand the microbiological assessment with the culture of the bronchoalveolar lavage (BAL), considering less frequent microorganisms in immunocompetent patients such as Pneumocystis carinii, fungi, Mycobacterium tuberculosis, etc. Moreover, a transbronchial biopsy (BTB) for histopathological evaluation of the lesions was performed. Bronchofibroscopy did not show any alteration in the airway mucosa or mucopurulent secretions. In the LBA microbiological study, all cultures were negative, including a new PCR for SARS-CoV-2. The pathological anatomy of the BTB included four fragments of evaluable lung parenchyma. It presented a temporally homogeneous pattern of interstitial thickening with mature-appearing edema and lymphocytic inflammatory infiltrate that was accentuated around vascular structures. No significant fibrosis was found, but type 2 pneumocyte hyperplasia with prominent microvacuolization of cytoplasm was evident. In addition, a single intra-alveolar fibroblast nest with the absence of exudative or inflammatory components was seen. No granulomas were observed. GMS (silver) staining showed no fungal structures or Pneumocystis. A mixed pattern of organized pneumonia (NO) and non-specific interstitial pneumonia (NSIP) was described (Fig. 1c). The characteristics of the lesions pointed to a possible toxic-drug cause.
After completing the study and with the diagnosis of flecainide pneumonitis, the withdrawal of the drug was decided, changing to diltiazem to control his baseline tachyarrhythmia. Corticosteroid treatment was started with 30mg of oral prednisone every 24h, tapering the dose in the following weeks. A week later, the patient's condition improved substantially, her cough and dyspnea disappeared, she had mild side effects of the corticosteroid treatment such as insomnia and anxiety that gradually disappeared with the progressive decrease in the dose. After 4 weeks, she was reevaluated in the outpatient clinic. The X-ray showed a decrease in the infiltrates, and some scar images on the right base (Fig. 1d). Respiratory function tests were also performed in which she had a normal CO diffusion of 104%. In the subsequent follow-up, the patient remained asymptomatic. She was able to withdraw the prednisone treatment and after 3 months she showed a complete resolution of the radiologic infiltrates (Fig. 1e).
Flecainide-induced pneumonitis is a rare entity as previously indicated. The symptomatology described in the different cases is usually subacute and insidious. The diagnosis is of exclusion ruling out other types of affections such as atypical infectious processes. In our case, the SARS-CoV-2 pandemic situation at world, made it necessary to rule out COVID-19 involvement.
Informed consentThe authors declare that they have the patient's consent for publication.
In this article, our hospital protocols on the publication of patient data have been followed and patient privacy has been respected.
FundingThere was no type of funding in this study.
Authors’ contributionsGuarantor of the paper: DFP. Conceptualization: DFP, STM. Anatomopathological evaluation: JGR. Radiological assessment: EP. Cardiological assessment: CC. Pulmonology evaluation: DFP, STM, SIC, DIF. Translation in English: DIF. Supervision: JGR, EP, DFP. Writing – review and editing: DFP, JGR, DIF, STM, SIC, EP, CC.
Conflicts of interestThe undersigned warrant that the article is original, does not infringe upon any copyright or other proprietary right of any third party, is not under consideration by another journal, has not been published previously and all the authors participated in the preparation of the manuscript. All authors have seen and approved the manuscript, contributed significantly to the work and declare that they have no conflicting interests that are relevant to this article.