Modern management of thoracic disease is dominated by ultrasound assessment with strong evidence supporting its use in many clinical settings, providing both diagnostic and procedural. Thoracic ultrasound is a pivotal step in the management of chronic lung disease and pulmonary vascular disease, in early assessment as in therapeutic monitoring. Development and validation of novel ultrasound biomarkers of activity and prognostic, especially those linked to advanced ultrasound techniques, are expected in the coming years. Assessing and treating respiratory muscle dysfunction is crucial for patients with both acute and chronic respiratory failure. To explore novel techniques, including imaging with ultrasound is important. Artificial intelligence (AI) excels at automatically recognizing complex patterns and providing quantitative assessment for imaging data, showing high potential to assist physicians in acquiring more accurate and reproducible results. Finally, a training system with structured proficiency and competency standards, about the use of TU is necessary. We offer our perspective on the challenges and opportunities for the clinical practice in other scenarios.
El tratamiento moderno de la enfermedad torácica está dominado por la evaluación ecográfica, con una sólida evidencia que respalda su uso en muchos entornos clínicos, proporcionando tanto diagnóstico como capacidad terapéutica. La ecografía torácica es un paso fundamental en el manejo de la enfermedad pulmonar crónica y la enfermedad vascular, tanto en la evaluación temprana como en el seguimiento terapéutico. Se espera que en los próximos años se desarrollen y validen nuevos biomarcadores ecográficos de actividad y pronóstico, especialmente aquellos vinculados a técnicas ecográficas avanzadas. La evaluación y el tratamiento de la disfunción muscular respiratoria es crucial para los pacientes con insuficiencia respiratoria aguda y crónica. Es importante explorar nuevas técnicas, incluida la obtención de imágenes con ecografía. La inteligencia artificial (IA) se destaca por reconocer automáticamente patrones complejos y proporcionar una evaluación cuantitativa de los datos de imágenes, mostrando un alto potencial para ayudar a los médicos a obtener resultados más precisos y reproducibles. Finalmente, es necesario un sistema de capacitación con estándares estructurados de competencia y habilidad sobre el uso de la ecografía torácica. Ofrecemos nuestra perspectiva sobre los desafíos y las oportunidades para la práctica clínica de la ecografía torácica en otros escenarios.
Many works in the literature evaluated the role of Thoracic Ultrasound (TU) in the study of several thoracic diseases, especially in the acute setting such as cardiogenic pulmonary edema, pneumoniae, acute respiratory distress, pleural effusion, pulmonary infarction and pneumothorax.1 In recent years, attempts have been made to extend the application of the method to chronic and more context specialized diseases such as chronic fibrosing pathologies and tumors.1–3 We offer our perspective on the challenges and opportunities for the clinical practice in other scenarios.
- 1.
Diagnostic indications:
TU as a non-invasive diagnostic instrument that has gained relevance in the evaluation of various chronic lung pathologies, such as fibrotic diseases, Chronic Obstructive Pulmonary Disease (COPD) and asthma.
Pulmonary fibrosisThere are different protocols for the performance of TU in the study of interstitial diseases. In these protocols, the exploration for the anterior and lateral thoracic regions is always performed with the patient in the dorsal decubitus position with the arms on the head, and in the sitting position for the exploration of the posterior areas. However, there is no agreement on the number of intercostal spaces to be explored or the type of probe used.4,5
Interstitial syndrome is defined by more than three comet tail artifacts (lines B) arising from the pleura line, visible in a frozen image in a longitudinal scan, with a distance no more than 7mm between two lines, in both lungs.7–10 The total number of B-lines is correlated with the extension of pulmonary fibrosis on High Resolution Computed Tomography (HRCT).11
The average distance between two adjacent B-lines can be an indicator of a particular pattern on HRCT, it is used comparing a pure reticular fibrotic pattern with a predominant ground glass pattern. In patients with predominant reticulation and honey combing, the distance between B – line is 7mm.12
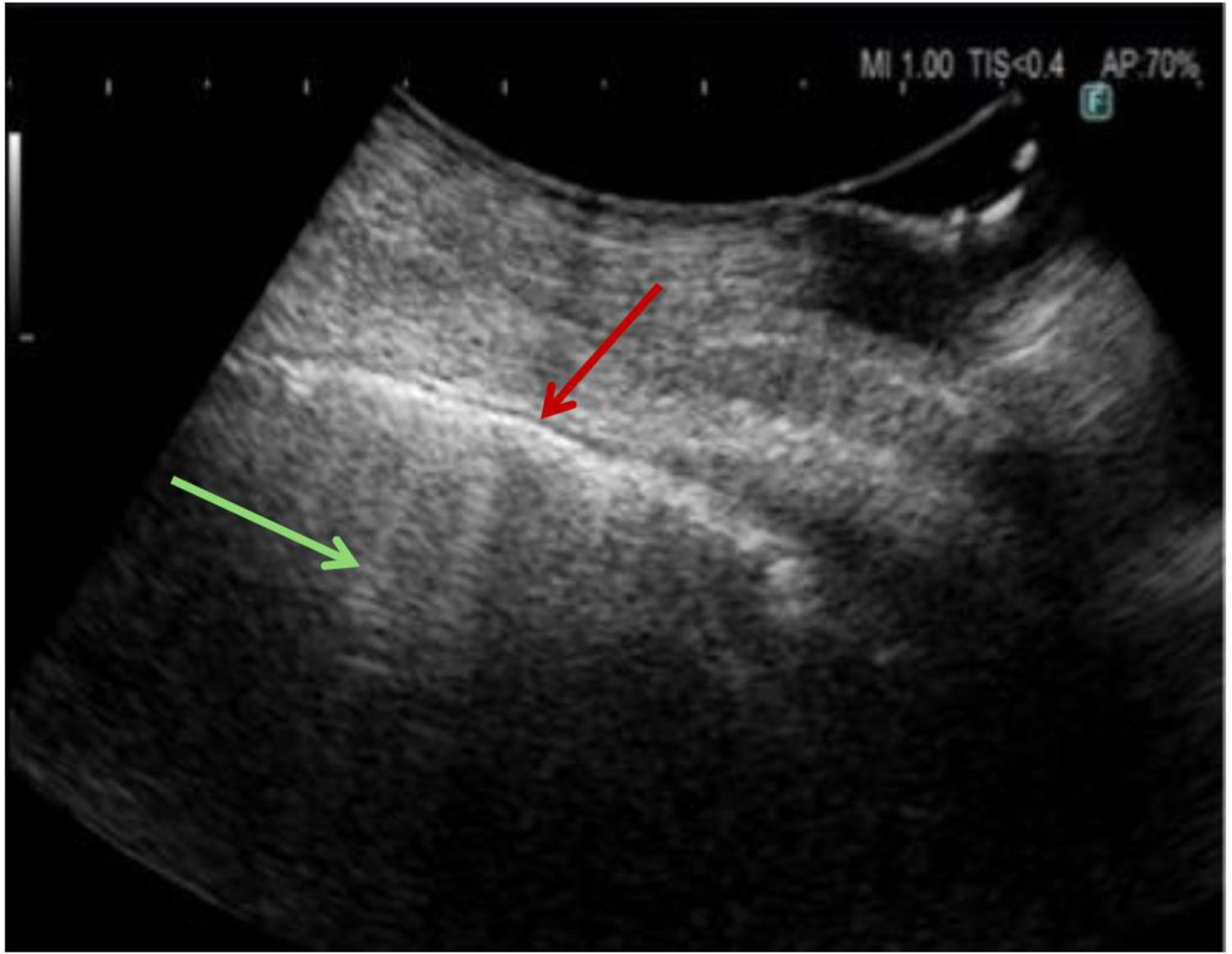
The other diagnostic sonographic finding is a thick, irregular, fragmented pleura line which is associated with subpleural fibrotic scars (Fig. 1). In healthy adults or in patients with pulmonary congestion, this line is thin. In fibrotic patients, this line is thickened>3mm, with an irregular fragmented appearance. In the most severe forms, pleural thickening is greater than 5mm.13
The distribution of the ultrasonographic artifacts can have a diagnostic value: an upper predominance of multiple B - lines associated with the thickening of pleura lines could help to exclude the idiopathic pulmonary fibrosis diagnosis in favor of a nonspecific pattern.13
The results of elastography in patients with interstitial lung disease show that in both TE mode (Transient Elastography) and pSWE mode (Point Shear-Wave Elastography), the wave velocity is significantly higher than in people without previous lung pathology. This has been demonstrated even at different wavelengths (100, 150 and 200Hz) for the TE mode. Published data on whether it can be useful for differentiating severity in diagnosed patients are conflicting. There are no data to suggest that it can be used in the long-term follow-up of interstitial diseases. Furthermore, the sensitivity and specificity of the cut-off points proposed in the published studies are very different, perhaps due to the heterogeneity of the cases considered and the methodologies and modalities employed. Larger studies with more homogeneous selection criteria and methodology are needed to assess the true usefulness of elastography in the diagnosis and follow-up of interstitial diseases.14–17
According to the findings discussed, TU is a useful and complementary tool in the management of the pulmonary fibrosis. Although it does not replace HRCT in terms of diagnostic accuracy, its practical advantages make it a valuable resource. Further research is needed to confirm its usefulness in detecting early changes in fibrotic lung, or for monitoring disease progression.
Chronic obstructive pulmonary diseaseMeasurements of diaphragm excursion and thickness, as well as lower limb muscles strength, size and thickness, may provide a safe, portable and effective alternative to radiation-based techniques in diagnosis and prognosis as well as tracking improvement postintervention in patients with COPD.18
Esmaeel et al. studied the clinical usefulness of chest ultrasonography in evaluating stable COPD patients and those exacerbated, focusing on diaphragmatic measurements and their correlation with spirometry and other clinical parameters.19 They performed a prospective case-control study, with 100 COPD patients divided into 40 stable COPD patients and 60 patients with exacerbation. Chest ultrasonography significantly showed high specificity, negative predictive value, positive predictive value, and accuracy in detecting pleural effusion, pneumonia, pneumothorax, and lung cancer. Diaphragmatic measurements were significantly lower among stable COPD subjects than healthy controls. Diaphragmatic thickness and excursion displayed a significant negative correlation with body mass index and the dyspnea scale, and a positive correlation with spirometry measures in both stable and exacerbated COPD.19
Diaphragmatic ultrasound can be used to assess diaphragmatic dysfunction in patients with COPD. Several studies have demonstrated its feasibility and high reproducibility in detecting diaphragm dysfunction in critically ill patients. It has also been proven to have a significant reference value in predicting the outcome of mechanical ventilation. It can also be useful as both a predictor of pulmonary rehabilitation efficacy and a real-time monitoring technique for respiratory muscle train-ing during such rehabilitation.20 We will develop these aspects in the section on therapeutic indications.
Wangüemert-Perez et al. studied if there were changes in diaphragmatic mobility and diaphragmatic thickness in 30 COPD patients after three months of treatment with indacaterol/glycopyrronium, showing a clear improvement in mobility and diaphragmatic thickness, but there were no significant changes in the diaphragmatic shortening fraction after treatment. This study concludes that TU can be useful for assessing the response to treatment with long-acting bronchodilators in these patients.21
For COPD patients in emergency or intensive care units, point-of-ca re ultrasound (POCUS) allows rapid assessment of lung status, helping in decision-making during exacerbations, respiratory failure, or in the evaluation of the response to therapy.22
Prior to endoscopic lung volume reduction with endobronchial valves may be useful in predicting the occurrence of subsequent pneumothorax, and also after the technique to assess its occurrence.23
AsthmaIt is not a standard tool for the management of this disease, but may be useful in certain cases such as: assessment of complications (in situations of severe exacerbation, it can help to assess the presence of complications such as pneumothorax or pleural effusion, especially if there is doubt about other diagnoses or coexistence of other diseases); it can also help to make a differential diagnosis against other causes of dyspnea such as pneumonia; and in intensive care units, where patients may be on mechanical ventilation due to a severe exacerbation of asthma, TU can be used to monitor lung dynamics and rule out complications.
Pulmonary vascular diseaseThis technique is emerging as a valuable tool in the diagnosis and management of pulmonary vascular diseases. The usefulness and indications for the use of TU in pulmonary embolism (PE) and pulmonary hypertension (PH) are discussed below.
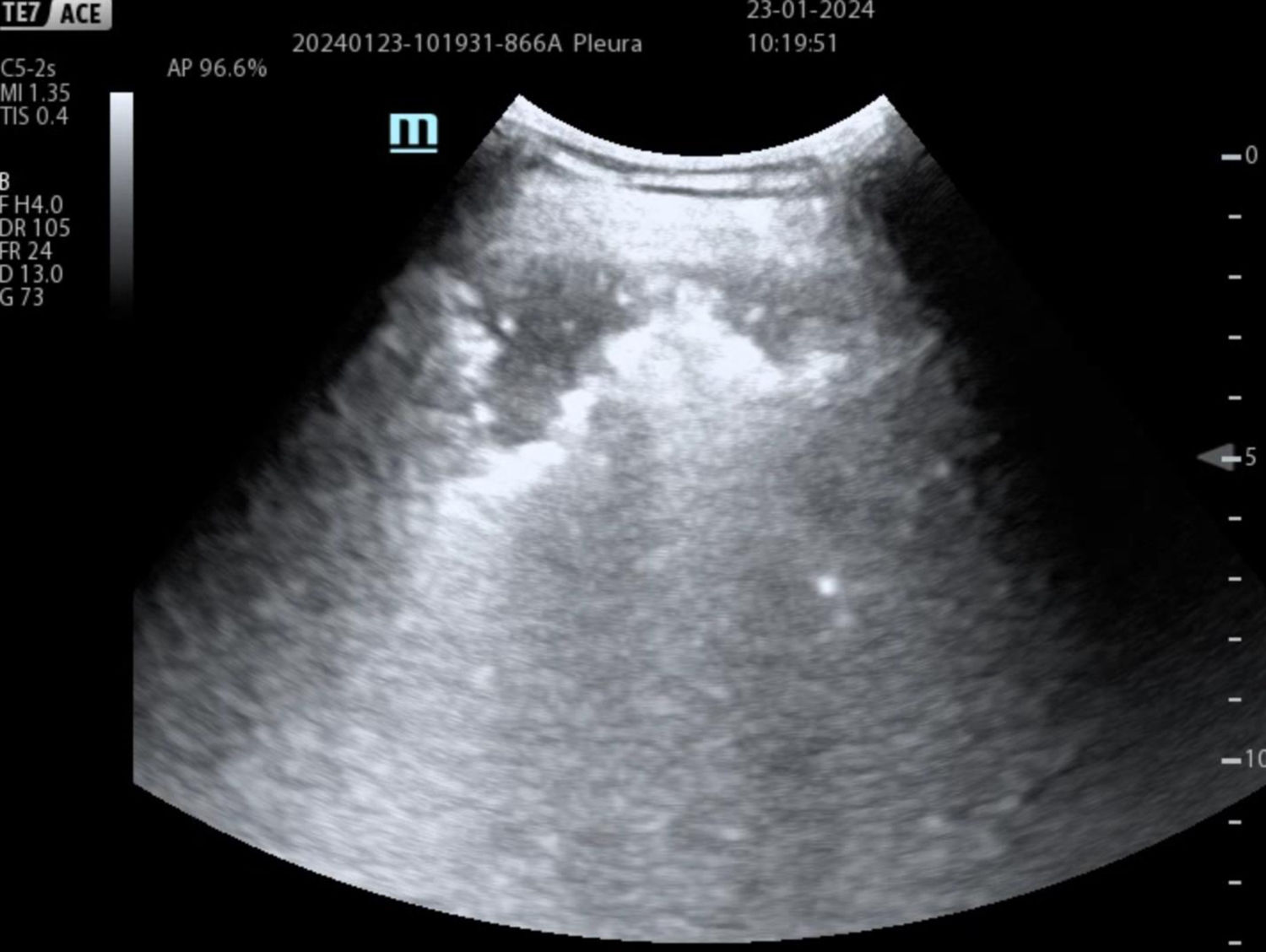
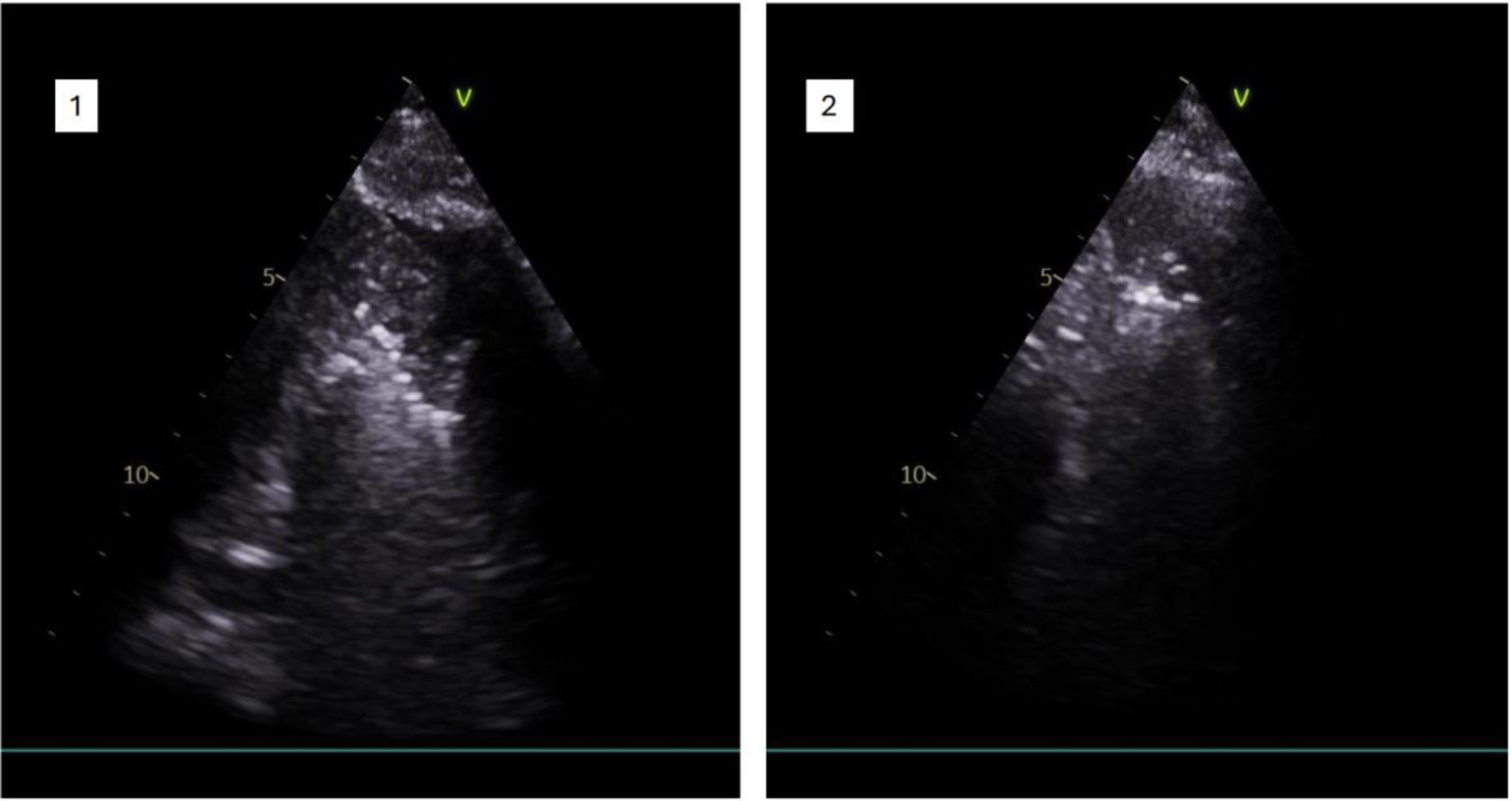
Pulmonary embolismRapid diagnosis of PE and correct treatment are key to survival and reduction of morbidity. Where CT is not available, in unstable patients with difficulty in mobilization,24 in patients with allergies to iodinated contrast, in chronic renal failure and in pregnant women,25 TU can make a diagnosis (pneumonia, pneumothorax, atelectasis, etc.) or guide the diagnosis of PE with a level of evidence A.26Table 1 describes the main ultrasound findings indicative of PE with compatible clinical features (Fig. 2).
Ultrasound features in pulmonary embolism.
| • Pulmonary infarcts are visualized as hypoechogenic lesions and generally of triangular morphology but also round or oval, with poorly demarcated borders, with pleural contact base (Fig. 2). |
| • The most frequent location is in the lower lobes and posterobasal areas.13 |
| • A central hyperechogenic lesion may be present in 20% of cases, indicating an air-filled bronchiole. |
| • The lesions do not present air bronchogram, unlike pneumonia. |
| • Color Doppler cannot detect pulmonary arterial flow, a finding known as “consolidation with low perfusion”. |
| • A congested thromboembolic vessel may be seen, which is called “vascular sign”. |
| • In most cases infarcts present effacement of the visceral pleural line, a finding useful in the differential diagnosis with other peripheral lesions (peripheral pneumonias or atelectasis) with hyperechogenic visceral pleura (Fig. 2). |
| • Pleural effusion is present in 20–50% of cases and is usually unilateral and occupies one third or less of the hemithorax.20 |
In a recent review and meta-analysis with 1916 patients, it had a sensitivity of 82% and a specificity of 89% for the diagnosis of PE.27 When in addition to TU we add cardiac ultrasound in the diagnosis of PE, the sensitivity increases to 91%.28
Moreover, it is well known that in more than one third of cases of PE thrombi come from lower limb veins. The Bedside Lung Ultrasonography in the Emergency Department (BLUE) protocol allows the diagnosis of PE based on positive venous ultrasonography in combination with TU exclusion of other causes of respiratory failure. The BLUE protocol require the mastery of signs indicating normal lung surface (bat sign, lung sliding, A-lines), pleural effusions (quad and sinusoid sign), lung consolidations (fractal and tissue-like sign), interstitial syndrome (lung rockets), and pneumothorax (stratosphere sign and the lung point) and the profiles have been designed for the main diseases (pneumonia, congestive heart failure, COPD, asthma, pulmonary embolism, pneumothorax).29 The BLUE protocol showed a sensitivity of 81% and a specificity of 99% for the diagnosis of PE.29
Therefore, the multi-organ (lung, heart, lower limb) use of bedside ultrasound may be of acceptable diagnostic value in patients with clinical suspicion of PE where computed tomography pulmonary angiography is not possible or contraindicated.30,31
Pulmonary hypertensionEchocardiography is an excellent method for the initial evaluation of the patient with a suspected diagnosis of PH, detecting elevated pulmonary artery pressure values, evaluating right ventricular function, and performing a differential diagnosis to detect underlying conditions, such as congenital heart disease, valvular pathology, or left heart disease, responsible for the presence of the disease. The differential diagnosis between pulmonary arterial hypertension (PAH) and PH due to heart failure with preserved ejection fraction is of great therapeutic relevance but can be difficult even if guided by a step-by-step approach considering cardiovascular risk factors, clinical examination, biomarkers, cardiac imaging, and eventual cardiac catheterization. At this point TU can be helpful: pulmonary congestion can be detected and semi-quantified by lung ultrasound imaging of the so-called B-lines. Its diagnosis may have prognostic relevance in patients with heart failure. D’Alto et al.30 evaluated the added value of TU to a previously reported echocardiography prediction score before and after a fluid test to improve the differential diagnosis between PH with heart failure with preserved ejection fraction and PAH. According to the authors, the results of the study may warrant further studies to validate echocardiography combined with TU and fluid challenge or exercise for differential diagnosis between these two entities.
Echocardiogram for pulmonologistsThe respiratory and cardiovascular systems are intricately connected, so much so that respiratory diseases can lead to cardiac dysfunction. The primary pathophysiological mechanism responsible for right ventricular (RV) involvement, in these cases, is hypoxemia.32 Hypoxemia triggers pulmonary vasoconstriction, increasing pressure in the pulmonary vessels. This PH progressively increases pulmonary vascular resistance, eventually leading to right-sided heart failure, which can be fatal for the patient.
PH associated with chronic lung disease is a common form of PH and exacerbates prognosis, shortening patient survival.33 COPD and/or pulmonary emphysema, as well as interstitial lung diseases, are the primary chronic respiratory conditions associated with PH (Group 3 of the clinical classification).34 In this group it's critical to correctly phenotype the type of PH, as these patients often have associated comorbidities, which makes the evaluation more complex. PH associated with pulmonary vascular diseases (PVD) includes pulmonary arterial hypertension (PAH), group 1 of the clinical classification of PH, and chronic thromboembolic pulmonary hypertension (CTEPH), which falls under group 4 of the clinical classification.34 PAH is a rare form of PH that classically affects young people, particularly women, with no associated comorbidities. However, more cases are now being diagnosed in individuals over 65 years of age with cardiovascular comorbidities, and the distribution between sexes is becoming more balanced.35 Although there is no curative treatment for this disease, recent pharmacological advances have significantly improved patient survival, with the goal of keeping patients in a low-risk state during follow-up.36 CTEPH is a severe and late complication of PE, with an incidence of 2.7% among patients who survive a thromboembolic event,37 being the only type of PH that has a potentially curative treatment.38
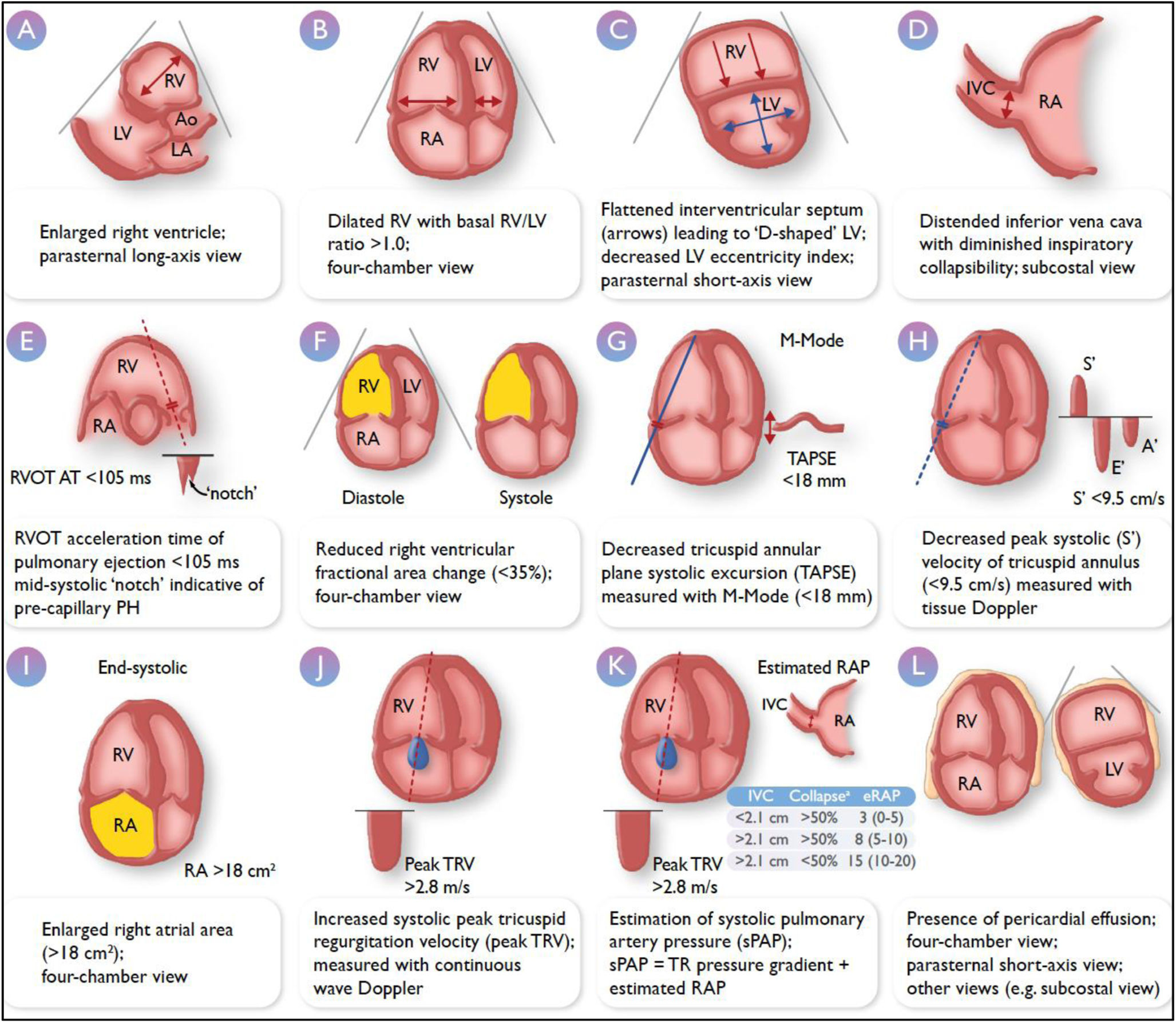
PH is thus a hemodynamic condition34 that must be confirmed through right heart catheterization, and a presumptive diagnosis must first be made using a non-invasive imaging technique due to the nonspecific nature of its symptoms. Transthoracic echocardiography is the imaging modality of choice,38,39 given its low cost, safety, availability, and portability. Echocardiography allows for the estimation of the probability that a patient with suspected PH truly has the condition,38,39 as well as the severity of the hypertension. Fig. 3 illustrates the echocardiographic parameters that should be assessed in PH.39 Considering these points, assessing RV function using echocardiography will be essential during the follow-up of these patients, and it is in this context that echocardiography performed by pulmonologists becomes relevant. The greatest utility will be seen in patients with PVD, where such evaluation may help in making rapid decisions regarding disease management. In patients with PAH, echocardiographic parameters of right heart function are part of risk stratification36 and are thus crucial for making therapeutic decisions. In patients who have experienced a PE and continue to experience respiratory symptoms after three months of proper anticoagulant therapy with no other underlying cause, CTEPH should be suspected, and the diagnostic algorithm should be initiated.36 Echocardiography plays a part in the initial evaluation. Additionally, a screening for CTEPH should be performed in patients who, despite being asymptomatic following a thromboembolic event, have risk factors for its development.40 Structured follow-up of patients with PE and early echocardiographic evaluation of RV function in the outpatient setting can prevent delays in diagnosing this condition and improve patient survival.41
Transthoracic echocardiographic parameters in the assessment of pulmonary hypertension. Adapted from Humbert M. European Heart Journal (2022) 00, 1–114. https://doi.org/10.1093/eurheartj/ehac237. Ao, aorta; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PH, pulmonary hypertension; RA, right atrium; RAP, right atrial pressure; RV, right ventricle; RVOT AT, right ventricular outflow tract acceleration time; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TRV, tricuspid regurgitation velocity. aRefers to collapse on inspiration.
In patients with chronic lung disease and suspected PH,33 echocardiographic evaluation will be more challenging for the pulmonologist, as these patients may have associated cardiovascular comorbidities that may contribute to multifactorial PH. Moreover, the quality of echocardiographic assessment may be suboptimal due to poor acoustic windows, altered cardiac positioning within the thoracic cavity, and interference from lung tissue surrounding the heart.
In conclusion, echocardiography performed by pulmonologists would provide added value within a dedicated unit for patients with PVD, aiding in the rapid decision-making process for the diagnosis and therapeutic management.
- 2.
Therapeutic indications
TU can be used by physiotherapists as a tool to facilitate the management of airway secretion retention, alveolar recruitment and ventilation/perfusion mismatching as well as for the evaluation of muscle function of the respiratory system (diaphragm, intercostal and abdominal muscles).42
Concerning the lung status and alveolar recruitment there are several aspects that we consider managing ventilation and choosing intervention techniques. There are several lung scores that allow to identify aeration levels and choose the best position and technique to apply. Secretion retention is a really challenging aspect that needs a good expertise level because it needs to put together the identification of static or dynamic bronchogram, consolidation, B lines presence or pleural effusion that allows the identification of the affected areas to optimize the goals of the intervention, choose the adequate techniques and evaluate its effectivity. Le Neindre et al.42 in a cohort study quantified that 62% of the physiotherapist changed their intervention when the TU scan was performed, which makes clear the importance of use in the physiotherapists daily clinical practice, as you can see in Fig. 5.
In the respiratory muscle study, physiotherapists can rely on the TU to the evaluation of the readiness for the extubation, the status of the diaphragm, its thickening fraction, excursion or even the speed of contraction.43 There are clear cutoff values in the diaphragm thickening fraction and excursion, and even if there are several ways of proceeding, it is a tool that can provide clinical information and guide in the application of inspiratory muscle training programs.44,45 Other respiratory muscles are starting to be monitored with ultrasound, the abdominal wall in a publication by Schreider et al. was monitored and a lower than 127% thickening fraction (rectus abdominal, abdominal transversus and internal oblique) during cough in the 72h after extubation was associated with increased reintubation risk.46 Intercostal muscles also can provide information, Truong et al. in a systematic revision found that decreased intercostal thickness was associated with increased mechanical ventilation duration, and increased critical care length of stay, compared to increased thickness and intercostal thickening fraction was found to have an excellent predictive ability of weaning outcomes.47
- 3.
Artificial intelligence applications for thoracic ultrasound
As it is already happening in other areas of medical imaging, Artificial Intelligence (AI) is now being applied to the analysis of TU data.2,48–51
In the original international consensus for point-of-care TU,2 the first statement is about AI-related technology for analyzing TU imaging.2
A first concept of interest is that AI could improve the diagnostic capacity of non-expert clinicians. In a recent work by Nhat et al.52 non-expert clinicians improved their performance from an average of 70–83% (p<0.001) when supported by our AI tool. The time-to-interpret clips improved form a median of 12.1–5.0s (p<0.001) and clinicians’ median confidence level improved from 3 out of 4 to 4 out of 4 when using our AI tool.52
A specific field of application is that of the evaluation of lung interstitial syndrome and thus the detection of vertical artifacts (B lines).10,11 Recent works tested the accuracy of AI in recognizing B lines from a deep learning algorithm.
TU is an important imaging modality used by emergency physicians to assess pulmonary congestion at the patient bedside. Some results showed that the area under the receiver operating characteristic curve ranges from 0.864 to 0.955 for the deep learning for detection and localization of B-Lines.53
In other prospective study, the objective was to compare the accuracy of AI versus real-time physician assessment lines B. The physician was 97% sensitive and 79% specific. The AI software was 95% sensitive and 64% specific. Both the physician and AI software were highly sensitive, though the physician was more specific.54
A number of studies have been conducted using AI to extract semi-quantitative parameters out of these images, for scoring, and classification in the context of the COVID-19 pandemic.55–58 Therefore, AI-based TU demonstrated an excellent agreement with HRCT validated COVID-19 pneumonia, even when used by not expert sonographers.
About the role of AI in the evaluation of pleural effusion, Tsai CH et al. demonstrated a deep-learning model capable of detecting the presence of pleural effusion on TU at an accuracy greater than 90%, when compared to an experienced operator.51
AI-assisted TU can help clinicians in interpreting TU features more accurately, more quickly and more confidently (Fig. 4). Future studies should explore the potential benefits of AI tools for the regulatory, ethical and issues of the clinical use of AI methods in different healthcare settings.
- 4.
Training and skills training in thoracic ultrasound
In 2018, an ERS monograph established that considering the evidence on the reduction of the risk of iatrogenic complications in pleural procedures carried out with TU, nowadays it would be indefensible not to carry it out before any intervention due to suspected pleural fluid, except in extraordinary circumstances.59 Consequently, the demand for ultrasound training has increased exponentially, and with it, the need to define robust, validated and assessment tools to ensure patient safety and clinical excellence.60
Currently, several institutions offer training courses and guidelines for assessing TU competence. The ERS, for example, offers up to six courses per year, which are highly demanded, the UK Royal College of Radiologists published its first ultrasound training recommendations in 2005 and, more recently, it has been released an update which outlines training requirements for performing TU-guided procedures.59 This document suggests that training should include both theoretical (including the physics of ultrasound, the principles of image formation, and the recognition of artifacts) and practical contents, and sets out three levels of competence based on a hierarchy proposed by the European Federation of Societies of Ultrasound in Medicine and Biology and supported by the British Medical Ultrasound Society (Table 2).61
Minimum training requirements for the practice of medical ultrasound in Europe.
| Level 1: practice at this level would usually require the following abilitiesTo perform common examinations safely and accuratelyTo recognize and differentiate normal anatomy and pathologyTo diagnose common abnormalities within certain organ systemsTo recognize when referral for a second opinion is indicated |
| Level 2: practice at this level would usually require the following abilitiesTo accept and manage referrals from Level 1 practitionersTo recognize and correctly diagnose almost all pathology within the relevant organ systemTo perform basic, non-complex ultrasound-guided invasive proceduresTo teach ultrasound to trainees and to Level 1 practitionersTo conduct some research in ultrasound |
| Level 3: this is an advanced level of practice, which involves the following abilitiesTo accept tertiary referrals from Level 1 and 2 practitionersTo perform specialized ultrasound examinationsTo perform advanced ultrasound-guided invasive proceduresTo conduct substantial research in ultrasoundTo teach ultrasound at all levelsTo be aware of and to pursue developments in ultrasound |
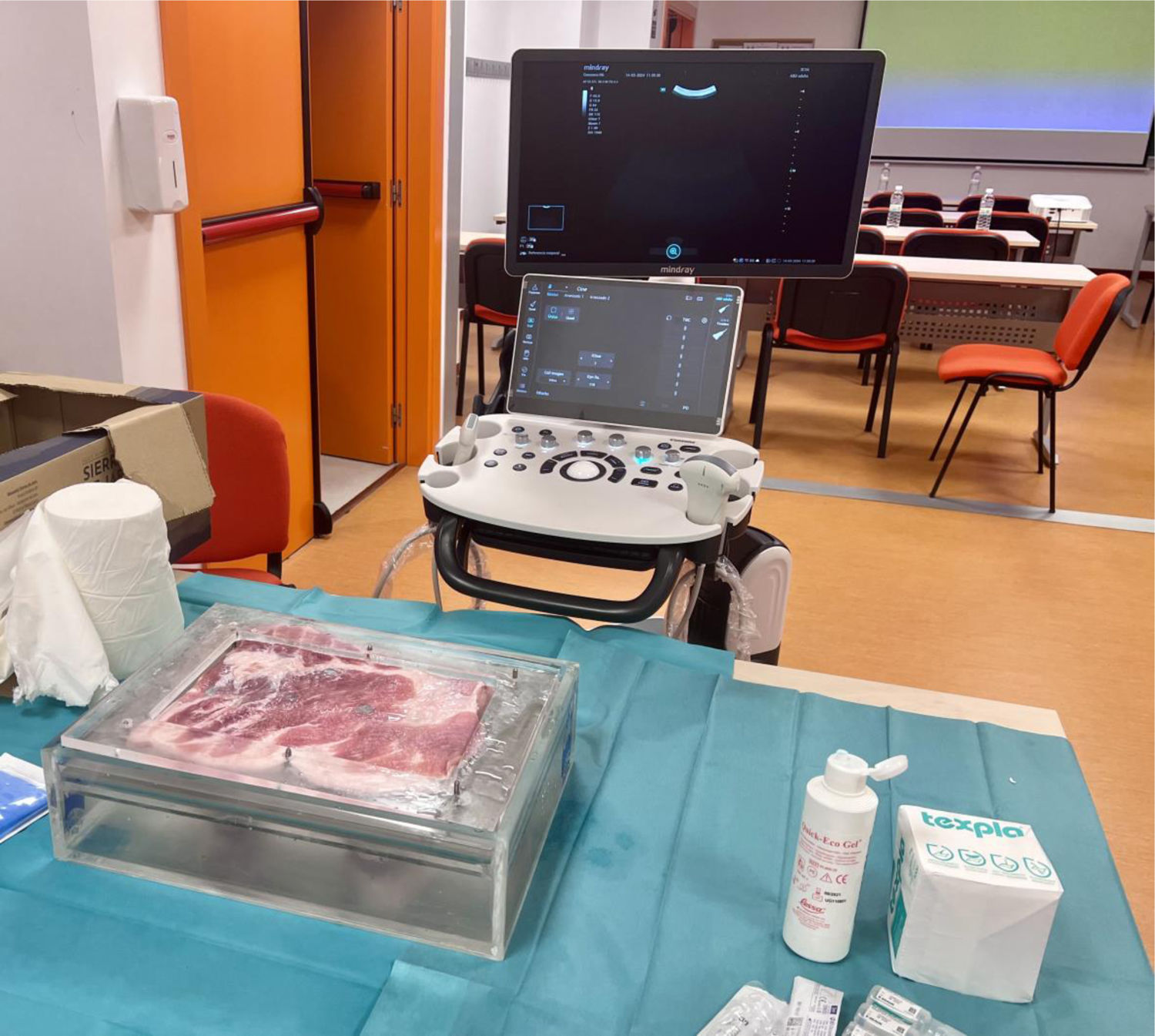
Most commonly, training is conducted at the bedside, during rounds with an experienced colleague, or in fixed-length courses although these are not the best learning conditions. On the one hand, clinical rounds require the availability of suitable patients and trained supervisors and can be a stressful and unsystematic environment; on the other hand, courses with a fixed duration do not guarantee that all students achieve an adequate competence as each trainee has a different learning pace. Hence, fixed-length courses or an arbitrary number of procedures performed/supervised cannot guarantee the acquisition of the competence. Alternatively, simulation-based or phantom-based training offers a standardized and stress-free learning environment where trainees practice their skills until they acquire the necessary competence as you can see in Fig. 6. Nonetheless, practical training must be preceded by solid and well-established theoretical knowledge.1
Assessing competencies is complex and multifaceted. The pyramid of competence, as defined by Miller in 1990, begins with “knows”, progresses through “knows how” and “demonstrates how”, and ends with “does”.62 Based on this, the assessment of competence in TU could be classified as follows: written tests assess “knows” or “knows how”. Objective, structured clinical examinations would assess “demonstrates how”; and current methods such as logbooks can satisfactorily measure “does”.59
In any case, the assessment should ensure the production of a good image with an adequate interpretation that leads to a correct clinical decision.59 There are several assessment tools, but all have limitations, so it is recommended to use personalized methods.63 In line with this, the most recent training options incorporate assessment methods that respect the different pace of competence acquisition by the candidate, thus advocating for mastery learning for all trainees regardless of their learning pace.64 For example, the ERS proposes a three-tiered TU training: the first is a theoretical module with online material and a final theoretical test. The second is a practical course on TU applied to routine clinical practice (online or on-site) which ends with the completion of a self-directed local training and the creation of a portfolio of cases and ultrasound clips on an online platform. The third level is a structured objective observed clinical examination (OSCE) during the ERS congress that must be passed to obtain the final certification.65 According to the students’ report, the use of TU increased as participants progressed through the course. Before taking the course, 44% of students used it several times a week, and 3 months after the completion, all participants used TU and more than 90% used it weekly.64
Despite the efforts made, with promising results, the standardization of competencies requires the intense and mutual collaboration of academic societies, and a supporting infrastructure for the design of accredited courses and the access to simulators and mentor supervision.
ConclusionsTU as an imaging modality has been extensively explored in recent years due to several advantages and it seems that this technology will gain even more space in thoracic disease. In this manuscript, we described and discussed the use of TU in the different fields. Appropriate validation in quality clinical trials will positively contribute to current patient management and improve prognosis and therapeutic options.
FundingThe authors have no funding.
Authors’ contributionAll authors should have made substantial contributions to all of the following (the conception and design of the study, or acquisition of data, or analysis and interpretation of data, drafting the article and revising it critically for important intellectual concept).
MBR, BRR: conceptualization, methodology, writing.
RM, TEH, RMRG, MMV: writing, formal analysis.
MBR, BRR: validation, supervision.
Conflicts of interestThe authors have no conflicts of interest.
Uncited references