Malignant pleural effusion (MPE) has become an increasingly prevalent complication in oncological patients, negatively impacting their quality of life and casting a shadow over their prognosis. Owing to the pathophysiological mechanisms involved and the heterogeneous nature of the underlying disease, this entity is both a diagnostic and therapeutic challenge. Advances in the understanding of MPE have led to a shift in the treatment paradigm towards a more personalized approach. This article provides a comprehensive review and update on the pathophysiology of MPE and describes the diagnostic tools and the latest advances in the treatment of this complex clinical entity.
El derrame pleural maligno (DPM) se ha convertido en una complicación cada vez más prevalente en los pacientes oncológicos, empeorando la calidad de vida y ensombreciendo el pronóstico de los mismos. Debido a los mecanismos fisiopatológicos involucrados y a la naturaleza heterogénea de la enfermedad subyacente, esta entidad representa un desafío diagnóstico y terapéutico. Los avances en la comprensión del DPM han originado un cambio en el paradigma del tratamiento hacia un enfoque más personalizado. Este artículo proporciona una revisión exhaustiva y una actualización sobre la fisiopatología del DPM, y describe las herramientas diagnósticas y los últimos avances en el tratamiento de esta compleja entidad clínica.
Malignant pleural effusion (MPE) is defined as the presence of neoplastic cells in pleural fluid.1 It can be associated with a primary pleural neoplasm (mesothelioma) or with secondary dissemination from any other location. Approximately 35% of MPE are secondary to lung cancer, which is present at diagnosis in 15% of patients, followed by breast cancer, present in 23% of MPE, and lymphomas in up to 10% of cases.2 The global incidence of MPE is estimated to be 70 per 100,000 individuals per year.3 Advances in the treatment of oncological pathology have resulted in increased survival. Management of this entity continues to be a clinical challenge, compromising quality of life. Prognosis depends on several factors such as the type of primary cancer, stage, and performance status. Two prognostic scoring systems have been validated for MPE: the LENT and PROMISE scores.4,5 Both systems combine clinical and biological variables but may not be valid in light of new targeted therapies for lung cancer and molecular subtypes.
Although diagnostic and therapeutic approaches to MPE have improved significantly in recent years, current treatment remains palliative, aiming to reduce symptoms through a variety of approaches, such as repeated thoracentesis, surgical or medical pleurodesis, and indwelling pleural catheters.
The aim of this review is to provide a comprehensive and updated overview of the pathogenesis, diagnosis, and treatment of MPE to guide clinicians in personalized management and optimize patient care.
Pathogenesis of malignant pleural effusionThe mechanism underlying the ability of the pleura to sustain homeostasis has been studied in depth.6 The quantity of normal pleural fluid (0.26mL/kg) depends on the balance of hydrostatic and oncotic pressure from the systemic and pulmonary circulation and the pleural space.6,7 An effusion manifests when fluid production exceeds the capacity of the lymphatic vessels to resorb fluid, resulting from factors such as elevated production, reduced resorption, or both.8,9
MPE is typically defined by the presence of cancerous cells, and with the exception of mesothelioma, its occurrence is predominantly the result of metastases in the pleural space.10,11 The mechanism of metastasis involves the invasion of the pleural space by tumor cells, mainly through the bloodstream, although direct infiltration or lymphatic spread may also occur. After initially invading the visceral pleura, diffusion to the parietal pleura occurs through tumor seeding along adhesions or by malignant cells floating in the fluid. Tumor cells adhere to the mesothelium, circumvent pleural immune defenses, infiltrate pleural tissue, and gain access to nutrients and growth factors.12,13 Therefore, the accumulation of fluid in the pleural space results from a combination of fluid extravasation from the hyperpermeable pleura or from tumor growth obstructing lymphatic drainage.14
MPE is characterized by a protein-rich fluid that comprises growth factors and cytokines, featuring proinflammatory molecules (IL-2, IL-6, and TNF), angiogenic factors (ANG-1 and ANG-2), and vascular permeability markers (VEGF, MMP, c-c motif, CCL, and OPN), as well as immunosuppressive substances such as IL-10.15 To initiate these processes, tumor cells activate transcriptional factors (nuclear factor NF-κB), signal transducer, and transcription-3 (STAT-3).12 Subsequently, the host cell IL-5 facilitates the influx of eosinophils and promotes myeloid suppressor cells in the pleura. In addition, mast cells release molecules (TPSAB1 and IL-1β) that enhance pulmonary vessel permeability.9,16 This interaction between the tumor and host cells establishes a microenvironment that facilitates tumor growth while inhibiting antitumor immune activity.14,15 Several recent translational studies have shown that the proliferation of cancer cell cultures is enhanced when cells are seeded in pleural fluid.17 In terms of genetics, genomic studies indicate that certain gene mutations (EGFR, KRAS, PIK3CA, BRAF, MET, EML4/ALK, and RET) are associated with a high risk of MPE formation. Specifically, KRAS mutations are more prevalent in distant metastases and EGFR mutations in tumors with regional metastatic infiltration.18
DiagnosisPleural fluid analysisThoracentesis is a low-risk procedure performed under ultrasound guidance. It helps to establish a definitive diagnosis of malignancy (diagnostic thoracentesis), relieve dyspnea caused by symptomatic effusion (therapeutic thoracentesis), or both. Pleural fluid samples should be sent for biochemical (5mL) and cytological (≥50mL) analyses.3 Malignant pleural fluids have a bloody appearance in approximately 40% of cases.19 These fluids are virtually always exudates, but one large series showed that 1.9% of 1527 MPE met Light's criteria for a transudate, most with a justifiable concomitant cause.20 The predominant cells in the pleural fluid differential white cell count are lymphocytes in about 85% of cases, pleural fluid glucose is ≤60mg/dL in 9%, pleural fluid pH is below 7.2 in 6%, and adenosine deaminase is ≥35U/L in 5%.19
Cytology of pleural fluid is the most straightforward method to reveal the presence of malignancy. The occurrence of positive results is influenced by factors such as the primary tumor, the quantity of individual specimens examined, and the method of sample processing.21 In a meta-analysis comprising over 6000 patients, the sensitivity of pleural fluid cytology for MPE was 58%, although the diagnostic yield was particularly low for lung squamous cell carcinoma (24%) and mesothelioma (29%).22 Maximal sensitivity may be achieved after examining two separate samples using both stained smears (Papanicolaou or Giemsa) and cell block (hematoxylin–eosin) preparations.23 Cell blocks allow the application of immunocytochemical tests to identify primary tumors invading the pleura and to differentiate between reactive mesothelial cells, mesothelioma, and adenocarcinoma. Thus, there are immunocytochemical markers for reactive mesothelial cells (e.g., desmin), mesothelioma (e.g., loss of BAP1 and/or MTAP, HEG1 clone SKM9-2), and carcinoma (e.g., claudin-4, EpCAM, and TTF-1).21,24–26 The diagnosis of mesothelioma is particularly challenging, although the combination of immunocytochemical tests (BAP1, MTAP) and fluorescence in situ hybridization (FISH) techniques (e.g., homozygous deletion of CDKN2A) has increased diagnostic sensitivity to 80–90%.27,28
Determining classical tumor markers in pleural fluid is indicative of malignancy but is not definitive. One study found that 41% of MPE with a false-negative cytological examination and a positive pleural biopsy had pleural fluid levels of CEA >45ng/mL or CA15-3 >77UI/L.29 Increasingly, pleural fluid (particularly the supernatant) is becoming a recognized source for liquid biopsy that allows the phenotypic characterization of tumors in a minimally invasive manner.30
Usefulness of pleural manometryPleural manometry refers to the direct measurement of pressure within the pleural space using anything from simple water manometers to electronic or digital devices.31,32 Potential clinical applications of pleural pressure monitoring include the diagnosis of non-expandable lung, prediction of pleurodesis success, and prevention of complications associated with large-volume thoracentesis.31
In non-expandable lungs, there is a greater decrease in pressure when the volume is removed. Pleural elastance, a change in pleural pressure divided by the change in the volume of pleural fluid drained, has shown to accurately differentiate non-expandable lung from normal lung,33 with a cut-off of 14.5cmH2O/l as the upper limit of normal.34 If pleural elastance is elevated (≥18cmH2O/l), the probability of pleurodesis failure is high.35,36 Therefore, pleural manometry may be recommended to guide the treatment of patients with MPE.
The use of pleural manometry may reduce the risk of complications associated with negative pressure (pulmonary edema due to re-expansion, chest pain, or exvacuo pneumothorax) during therapeutic thoracentesis; however, there is little evidence to support this approach. Several studies have failed to demonstrate that pleural manometry during large-volume thoracentesis reduces the risk of serious complications, discomfort, or breathlessness.37–39
Imaging techniquesUltrasonographyThoracic ultrasound (TUS) is a noninvasive tool that is cost-effective, portable, and readily available. It allows real-time image acquisition without radiation exposure.40,41 TUS is highly sensitive for detecting pleural effusion (PE). It can also identify the presence, localization, and characteristics of PE and allow the visualization of adjacent structures.42
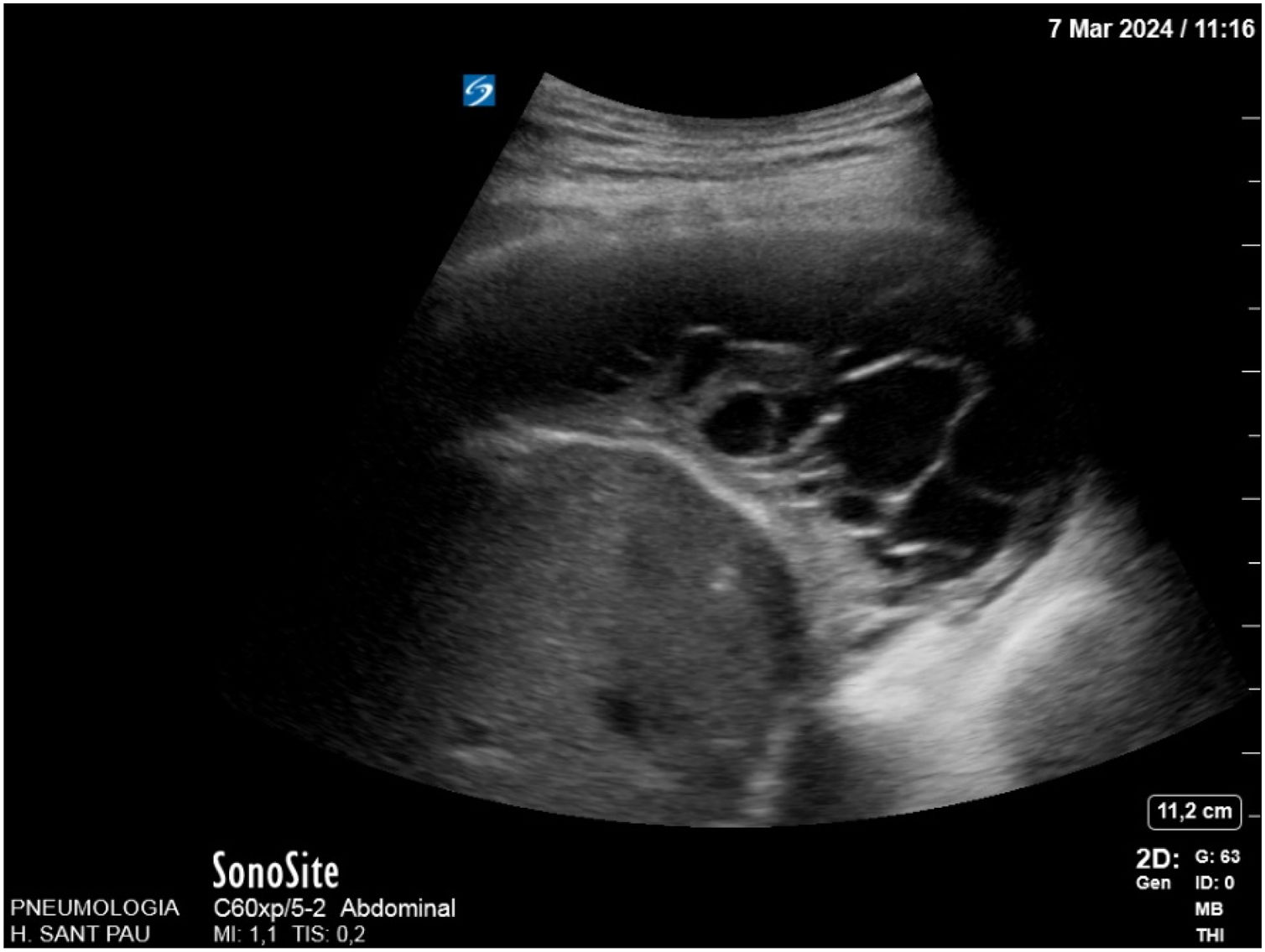
TUS plays a crucial role in the diagnosis, management, and monitoring of MPE. It is not only accurate, but also provides guidance for procedures, enabling detailed evaluation of the peripheral pleura in the presence of pleural effusion.3 TUS can reveal fibrin septa (Fig. 1) or loculations,43,44 pleural thickness, and pleural metastases.45 Furthermore, it can identify small PE and locate the best site for pleural catheter placement. It can also be useful for evaluating lung re-expansion.45 International guidelines recommend ultrasound guidance when performing thoracentesis to reduce the risk of complications,3,40,45 especially in loculated collections.44 Regarding pleural thickening or nodularity, ultrasound-guided pleural biopsy increases diagnostic accuracy3,46,47 and is highly sensitive.45,46
TUS has demonstrated reasonable sensitivity and high specificity for distinguishing malignant from benign effusions.48,49 Certain specific ultrasound characteristics have been associated with malignancy, such as pleural/diaphragmatic nodules, pleural thickening >1cm, diaphragmatic thickening >7mm, visceral pleural thickening, liver metastasis, and the absence of lung air bronchogram signs.48,50 The best predictors of malignancy are pleural or diaphragmatic nodularity (Fig. 2),3,40,43,50 but they have not proven useful as ruling-out tests for malignancy.40 Moreover, the detection of pleural thickness >15mm in B-mode shows high sensitivity for the diagnosis of malignancy.3,51
New modalities such as contrast-enhanced ultrasound (CEUS),51 M-mode and 2D-shear wave elastography52 could be helpful for differentiating benign from malignant pleural lesions and assessing lung re-expansion.44,53,54
Radiological signsChest X-rayThe following chest radiographic signs may indicate MPE55,56 (Fig. 3):
- -
Pleural effusion: A lateral radiograph can detect up to 50mL of pleural fluid. The possibility of malignant pleural disease should be considered when the liquid has a loculated morphology, when the distribution is unilateral or asymmetrical, when the amount is massive, or in the event of recurrence after drainage.
- -
Pleural thickening: Focal or diffuse nodular thickening of the pleura. Widening of the mediastinal silhouette can indicate thickening of the mediastinal pleura.
- -
Displacement of the mediastinal structures: A radiological sign that may present alone or in association with those previously described.
Pleural mesothelioma. (A) Chest radiograph showing massive left pleural effusion, thickening of the parietal pleura (white arrow) and left mediastinal widening (black arrow). (B) Chest CT shows diffuse pleural thickening involving the mediastinal pleura (black asterisk), and loculated pleural effusion (white asterisks).
Contrast-enhanced chest computed tomography (CT) is superior to conventional radiography in detecting pleural effusion and thickening. Furthermore, it enables better assessment of the pulmonary parenchyma and mediastinal structures.
- -
Pleural effusion: It allows further characterization of the liquid. Septations, elevated densitometric values (Hounsfield units),57,58 and loculations are radiological signs suggestive of pleural malignant disease.
- -
Pleural thickening: Features highly suggestive of malignancy are nodular pleural thickening, pleural irregularity, mediastinal pleural involvement, circumferential pleural thickening, and pleural thickness >10mm.
Although dual-energy CT is a promising tool for the evaluation of pleural lesions, its role in clinical practice has yet to be determined.59,60
FDG PET scanAs most malignant tumors have increased [18F]FDG uptake, [18F]FDG PET/CT can be useful in differentiating between benign and MPE.61,62 To differentiate benign from malignant lesions, Yang et al.63 developed and validated a PET-CT score, which showed a sensitivity of 89.7% (95% CI: 75.8–97.1%) and a specificity of 88.6% (73.3–96.8%). They found several patterns in the presentation of [18F]FDG uptake by MPE, including linear, nodular, and encasement (Fig. 4). Most cases of mesothelioma have an encasement pattern, but other malignant tumors such as lung cancer and lymphoma may also present this uptake pattern.64 One potential application of [18F]FDG PET/CT in the context of MPE is to guide biopsy procedures by identifying the region with the highest accumulation of [18F]FDG.65 [18F]FDG PET/CT can be used to assess the extent of malignant tumors with pleural involvement and will help monitor the response to therapy.66 Nevertheless, abnormal pleural FDG uptake is not specific for MPE. Inflammatory and infectious disorders, and pleurodesis may result in elevated [18F]FDG uptake.65
Image-guided pleural biopsyPleural biopsy (PB) is the gold standard for diagnosing MPE.67,68 Image-guided biopsies can be performed using CT or TUS, and this method has a high diagnostic yield (70–83%)69,70 (Fig. 5).
When pleural thickening and/or pleural lesions can be identified and targeted for biopsy, the sensitivity is significantly higher.71 If pleural thickening is >1cm, the diagnostic sensitivity of CT-guided PB is comparable to that of thoracoscopy.72,73 Both modalities show a good diagnostic yield, with TUS (84%) and CT guidance (76–100%) accompanied by low complication rates.70,73
Image-guided PB is particularly useful in adhered lungs that render medical thoracoscopy unsuitable, and in well-selected cases, it can provide a high diagnostic yield in the hands of trained clinicians.74
TUS also offers some advantages pertaining to patient pathways and waiting times. It usually takes less time than CT-guided PB. Furthermore, it can be conducted by physicians during the initial consultation with the patient, without requiring CT and without subjecting patients to ionizing radiation exposure.
Secondary spread from pleural metastases is more likely to initially be found in the lower thoracic parietal pleura. Ultrasound examination may help to identify a more suitable lower location for sampling. Botana-Rial et al. found that the sensitivity of US-assisted PB biopsies in MPE increased by >17% in subjects with MPE compared to closed PB without the asisitance of TUS.75
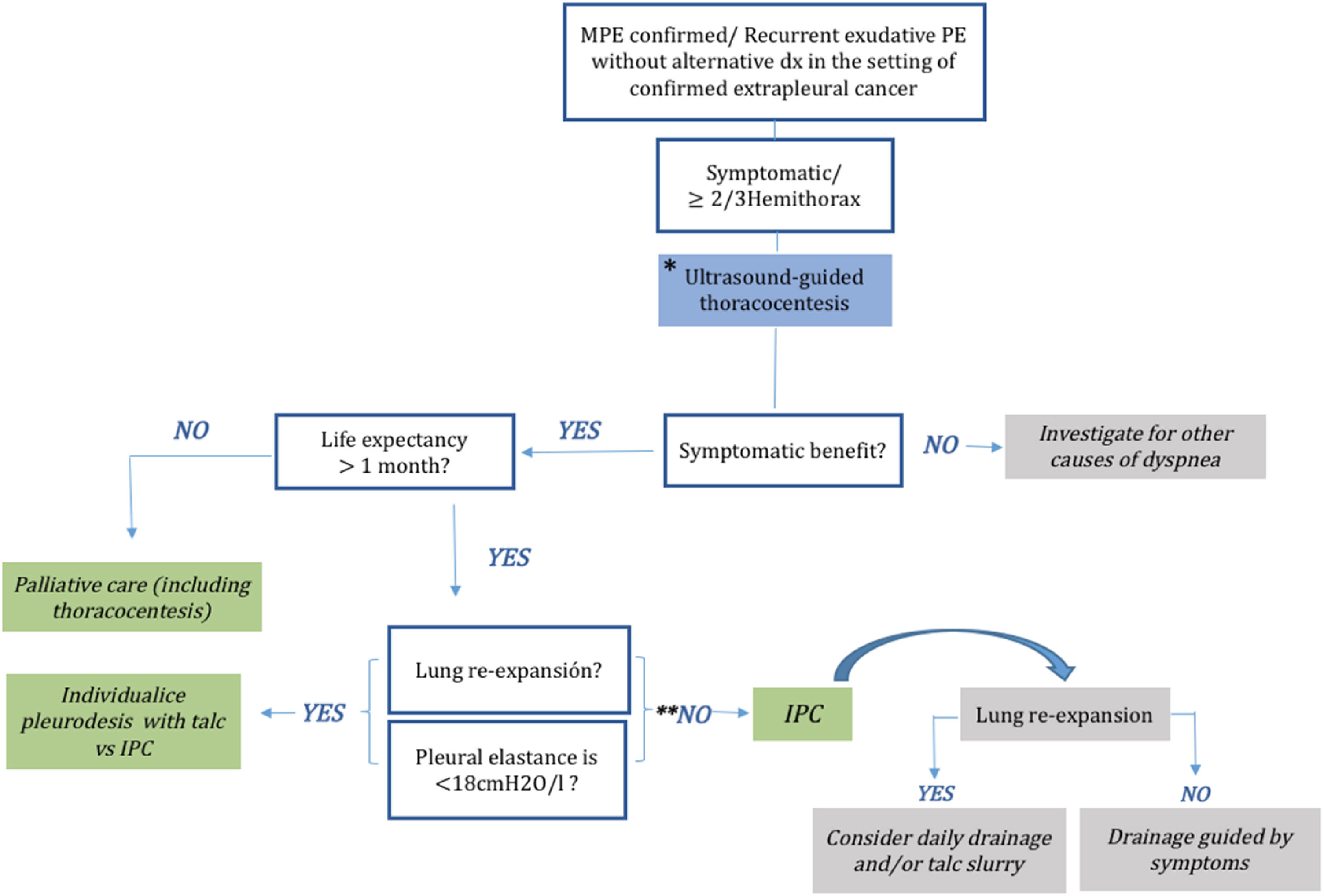
Management approachIndwelling pleural cathetersIndwelling pleural catheters (IPCs) are fenestrated silicone tubes. They are tunneled and fixed subcutaneously using a profibrotic polyester cuff, and have a one-way valve as the external endpiece. The drainage valve of an IPCs is an important component of the catheter that enables intermittent drainage at home via a user-friendly tube.91 In 1999, Putnam et al. published the first randomized trial comparing pleurodesis to IPC for MPE and found that patients with IPC experienced fewer in-hospital complications and a similar improvement in quality of life compared to patients with pleurodesis; however, they had a lower rate of spontaneous pleurodesis and higher rates of late complications.92 Currently, this type of drainage is mainly used to manage recurrent symptomatic MPE, aiming to improve quality of life by offering long-term symptom control with the same effectiveness as talc pleurodesis.45 IPC may alleviate dyspnea in 95% of patients with MPE.93 It was initially indicated when the lung did not expand and for patients with symptomatic and loculated fluid accumulation after a failed attempt at pleurodesis.94 IPC and chemical pleurodesis have demonstrated a similar improvement in symptoms. IPC can be inserted in the outpatient setting and produces spontaneous pleurodesis in 24–58% of patients with MPE. Furthermore, it is associated with fewer requirements for additional pleural interventions.93,95–98 The IPC drainage schedule is usually determined based on the symptoms. To achieve earlier spontaneous pleurodesis, daily drainage has been compared with drainage every other day, or symptom-guided drainage. Previous studies have shown that early pleurodesis is achieved with daily drainage, but without differences in quality of life, performance status, or patient satisfaction compared with other drainage intervals.97,99 In addition, in the lung that is not trapped, instillation of talc through the IPC produces spontaneous pleurodesis earlier. Complications of IPC are infrequent, and include catheter malfunction, infection, and symptomatic loculation. Infections such as empyema occur in approximately 5% of cases, and mortality related to such infections is less than 1%,100,101 including patients undergoing oncological treatment in whom the IPC can be placed at any time during oncological treatment without altering the efficacy and safety of IPC.102 Unless drainage is inadequate, most catheter-related infections can be treated by leaving the IPC in place, supplemented with systemic antibiotic therapy and intrapleural enzyme therapy through the catheter.103 Another complication is symptomatic loculation, which is treated with intrapleural enzyme therapy.104 The recently published European and American guidelines state that both IPC and pleurodesis are definitive first-line interventions for symptomatic MPE with expandable lungs, and IPC is also recommended for patients with non-expandable lungs or those with failed pleurodesis44,105 (Fig. 6).
PleurodesisPleurodesis refers to the process of obliterating the pleural space through the induction of pleural inflammation and fibrosis. This is accomplished through the use of a sclerosant or manual abrasion (mechanical). Mechanical pleurodesis, such as pleural abrasion, may be an option for patients with recurrent MPE. However, this procedure requires a surgical approach (thoracoscopy). Mechanical pleurodesis could be technically difficult if previous attempts at nonsurgical pleurodesis are partially successful or if the effusion is significantly loculated.83 It can also be combined with chemical pleurodesis; however, it is unclear whether this increases the efficacy of the procedure. The presence of vascularized lesions in the pleura may increase the risk of bleeding after abrasion. In contrast, in addition to thoracoscopy, chemical pleurodesis can be performed through an IPC or chest tube. Numerous agents have been used for chemical pleurodesis (tetracycline derivatives, silver nitrate, iodopovidone, bleomycin), with talc currently being the agent of choice.46 The most frequently described complications are chest pain, dyspnea, and fever. To minimize the risk of these complications, talc must be free of contaminants and have a particle size greater than 15μm.76 The average administered dose of talc is 4g. Administration can be either sprayed through a thoracoscope (talc poudrage) or suspended in saline via a chest drain (talc slurry). There are no differences between the two forms of administration, and the complication rate and improvement in symptoms are also similar.77,78 In general, the success of pleurodesis in terms of reducing the recurrence of pleural effusion 30 days after pleurodesis ranges from 60 to 90%,79–81 whereas the relapse rate in long-term survivors is 50% at six months of pleurodesis.81,82 A crucial point to bear in mind is that to ensure optimal pleurodesis, the pleural space must be as dry as possible prior to the application of a sclerosant agent.
Pleurodesis is typically recommended for symptomatic patients with a life expectancy of more than one month, provided they have shown clinical improvement and pulmonary re-expansion after a previous therapeutic thoracentesis (Fig. 7).
Other surgical approachesShuntPleuroperitoneal shunting is rarely used in patients with refractory MPE. It usually requires thoracoscopy and general anesthesia; however, shunts may also be placed using interventional radiological techniques.84–86
PleurectomyTotal pleurectomy with decortication is a highly invasive procedure that requires thoracotomy in most cases. It is technically challenging, with significant morbidity and mortality, and a long recovery time. Additionally, there is a paucity of evidence to support its use. It is indicated in selected cases that allow for good surgical conditions and long expected survival. Partial pleurectomy/decortication can be performed by thoracoscopy. Despite its greater technical difficulty, this approach leads to quicker recovery and less pain than thoracotomy.87–90
Pharmacologic interventionsUpdate in oncological treatmentAdenocarcinoma is the most frequent subtype of lung cancer associated with MPE. Around 50% of the Caucasian population and up to 70% of the Asian population present some driver molecular alterations that could potentially benefit from targeted therapies with high, rapid, and deep responses.106,107 Approximately 65% of patients with MPE with oncogenic driver alterations present with an EGFR mutation.108 In these patients, treatment with osimertinib results in an objective response rate (ORR) of 80%, with a time to response <6 weeks in 69% of patients and a median duration of response (DoR) of 17.2 months.109 A retrospective series of patients with EGFR mutations who received first-line osimertinib showed a lower ORR in patients with MPE than in those without MPE (58% vs. 71%), although this difference was not statistically significant (p=0.443). Additionally, there were no differences in progression-free survival (PFS) (19.8 months in both groups, p=0.693).110 Beyond the EGFR mutation, there are no large series on the specific response to MPE with other oncogenic driver alterations such as MET exon 14 skipping and BRAF mutation or ALK, RET, or NTRK fusions, although treatment with tyrosine kinase inhibitors (TKI) offers ORRs of 55 and −85%.107
Oncogenic driver alterations are not exclusive to non-small cell lung cancer, as approximately 15% of breast cancer patients present Her2 overexpression or amplification, and treatment ORRs with dual anti-Her2 blockade plus chemotherapy reach 80% with a median DoR of 20.2 months.111 Another example is melanoma, in which more than 50% of patients present with a BRAF V600 mutation, and treatment with BRAF/MEK inhibitors results in ORRs of 63%.112
Regarding chemotherapy (ChT), patients with highly sensitive tumors, such as germ cell tumors and lymphomas, reach a high cure rate despite the presence of MPE. In other tumors, such as small cell lung carcinoma or ovarian carcinoma, despite not having radical intention, ChT achieves ORRs of up to 60% and 75%, respectively.113,114
In recent years, immune checkpoint inhibitors (ICI) have become a part of the standard oncologic treatment, either as monotherapy or in combination. The combination of two ICIs achieved 60% ORR in melanoma,115 the combination of ICI plus anti-vascular endothelial growth factor (VEGF) achieved 56% ORR in renal carcinoma,116 and the combination of ICI with an antibody–drug conjugate achieved 68% ORR in urothelial carcinoma.117
In selected patients in whom a rapid and deep response is expected from oncological treatment, close monitoring of pleural effusion in the first weeks of initiating systemic therapy could help in decision-making concerning pleural management (IPC or pleurodesis) to prevent recurrent thoracentesis.
Palliative careDyspnea, pain, cough, and anxiety are the physical and emotional symptoms most frequently associated with MPE.13,118 Pharmacological treatment, which typically includes the use of opioids, plays a crucial role in effectively alleviating these symptoms and improving the quality of life.119–127
Although morphine is the most well-studied opioid, some publications support a similar effectiveness for opioids such as oxycodone, fentanyl, or methadone.128–130
The use of benzodiazepines combined with opioids may help improve dyspnea. Benzodiazepines should be considered for patients with accompanying anxiety.131,132
If major opioids are not used, cough can be treated133,134 specifically with minor opioids (codeine, dihydrocodeine). The use of levodropropizine or sodium cromoglycate is also effective.
To ensure optimal symptom control and quality of life, regular assessment and monitoring of the patient's response to treatment are essential.120,123,126,127
For better scientific evidence, research with larger numbers of patients, using standardized protocols and quality of life measures, would be necessary.126,131,134
ConclusionsMPE is a complex condition that requires a comprehensive approach to establish an accurate diagnosis, optimize oncological treatment, and improve patient quality of life. Management of MPE is challenging and mainly focuses on symptom relief. It involves early assessment of definitive pleural techniques, such as pleurodesis and tunneled pleural catheter insertion, to achieve initial symptom control. It is crucial to conduct follow-ups in multidisciplinary units to monitor patients, assess treatment responses, manage disease progression, and adjust management as needed. Further research is needed to evaluate these controversies and to establish the most beneficial treatment modality.
FundingThis study did not receive any type of funding.
Authors’ contributionsConceptualization and supervision: Ana Pardessus, Virginia Pajares.
Writing – original draft: Ana Pardessus, Albert Rafecas-Codern, José M. Porcel, Pere Serra-Mitjà, Lucía Ferreiro, Maribel Botana-Rial, Cristina Ramos-Hernández, José Manuel Brenes, Lydia Canales, V. Camacho, Beatriz Romero-Romero, Juan Carlos Trujillo, Elisabeth Martinez, Enrique Cases, Andrés Barba, Margarita Majem, Ernest Güell, Virginia Pajares.
All authors reviewed and approved the final version of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.













![[18F]FDG PET/CT with left pleural effusion in the CT study (A and B). The [18F]FDG images (C and D) and the [18F]FDG PET/CT fusion images (E and F) present increased glycolytic metabolism with an encapsulation pattern. Pathological diagnosis: mesothelioma. [18F]FDG PET/CT with left pleural effusion in the CT study (A and B). The [18F]FDG images (C and D) and the [18F]FDG PET/CT fusion images (E and F) present increased glycolytic metabolism with an encapsulation pattern. Pathological diagnosis: mesothelioma.](https://static.elsevier.es/multimedia/26596636/0000000600000004/v2_202503100908/S2659663624000523/v2_202503100908/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



