Malignant pleural effusion (MPE) is the most common cause of recurrent exudative pleural effusions (PE). Indwelling pleural catheters (IPCs) offer an alternative to bedside or thoracoscopic talc pleurodesis for controlling persistent symptomatic effusions.1 IPC, which is a silicone tube placed in the pleural cavity and tunneled subcutaneously, allows for ambulatory management, thereby minimizing hospital stay. The external portion of the IPC is attached to vacuum bottles for drainage.2
A meta-analysis of 1348 patients with MPEs treated with IPCs revealed that 95.6% had symptomatic improvement and 45.6% achieved spontaneous pleurodesis (SP) after a median of 52 days.3 Following successful IPC placement, no further ipsilateral pleural procedures were required in 92% of 279 MPE cases from another series.4 However, IPC drainage using commercially available vacuum bottles is expensive and often financially prohibitive in developing countries. Therefore, looking for an alternative drainage strategy using the IPC, but with manageable cost, is necessary5 as we report in the following experience.
MethodsA prospective study was performed from January 2016 to June 2018 at a tertiary University Hospital in Monterrey, México. It included patients with MPEs and chronic benign PEs refractory to medical treatment and thoracenteses. An IPC was indicated for symptomatic patients with contraindications for pleurodesis (unexpandable lung) and a predicted survival of at least 1 month, and also for those who preferred the IPC to classical pleurodesis after a discussion of the pros and cons. The presence of a non-expandable lung was evaluated on a post-therapeutic thoracentesis chest radiograph, and it was defined as <50% pleural apposition based on subjective visual estimation. The aim of IPC placement was the improvement of performance status with no need for further pleural intervention and, secondarily, the achievement of a spontaneous pleurodesis (SP) leading to IPC removal. Effusions were classified as malignant on the basis of a positive pleural fluid cytology or histology, whereas the diagnosis of benign effusions depended on well-established clinical criteria.6
Before IPC insertion (PleurX™, Becton Dickinson), patients were subjected to a clinical (including Eastern Oncology Cooperative Group – ECOG – and Karnofsky scales), analytical and thoracic ultrasound (TUS) evaluation. The latter determined the pleural fluid pattern (anechoic, echogenic, and complex septated or non-septated), presence of pleural thickening and optimal point of entry for IPC placement. Pleural drainage was performed either with vacuum bottles (PleurX drainage kit, 500mL) or manually depending on the patient's economic resources. In Mexico, the approximate cost of one 500mL vacuum bottle is 80 dollars while that of manual drainage is only 3. Manual drainage was performed using a 14G angiocatheter (without the introducer needle) which was inserted into the outer end of the IPC (valve) and connected through a three-way stopcock with a 50mL syringe. The manually aspirated fluid was drained into a simple non-sterile plastic bottle. Both vacuum and manual drainages were performed twice weekly by a nurse or a family doctor in the outpatient setting. A monthly clinical and TUS follow-up was conducted by a pulmonologist. The IPC was removed when the amount drained was less than 50mL for three consecutive drainages and a TUS demonstrated either the absence or a minimal quantity of fluid; a situation which was presumed to represent a spontaneous pleurodesis (SP).
Vacuum bottles and manual drainage groups were compared with the chi-square, Student's t or Mann–Whitney U tests, as appropriate. The research local committee approved the study protocol (Ref. No. NM17-00006).
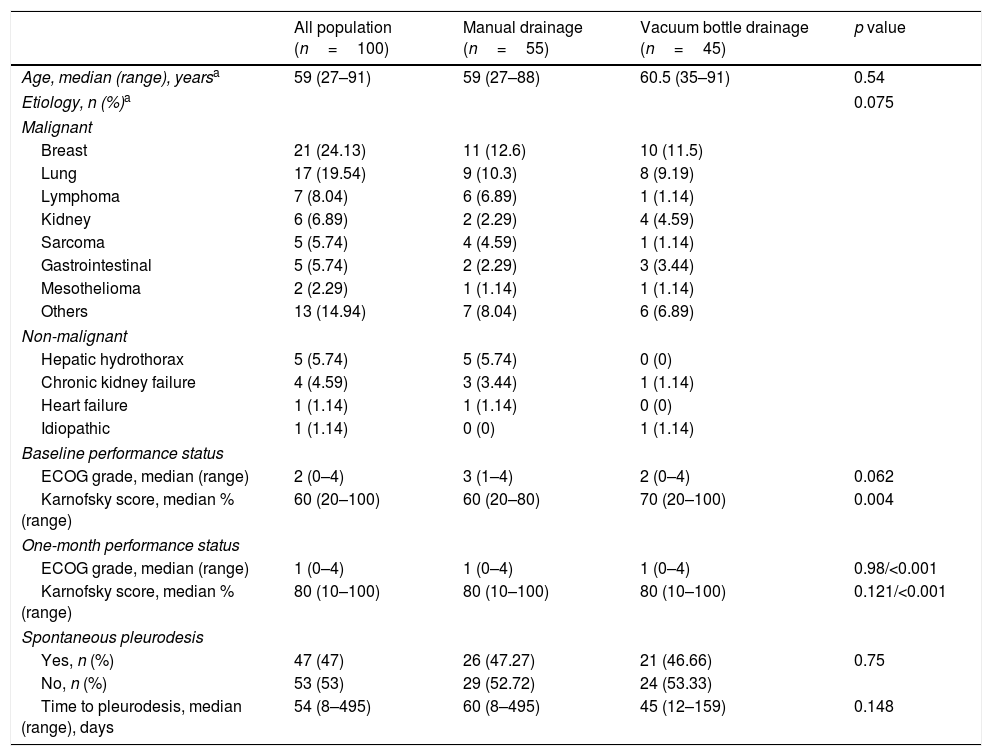
ResultsA total of 100 IPCs were inserted into 87 patients. Eleven (12.6%) required bilateral IPCs and one needed three IPCs. The median age population was 59 years (range 27–91); 40 patients (46%) were male and 47 (54%) female (54%). There were 76 patients (87%) with MPEs and 11 (13%) with benign conditions. The most common indications for IPC placement were breast cancer (21 patients, 24%) and lung cancer (17 patients, 19.5%). Forty-five percent of IPC drainages were performed using vacuum bottles and 55% manually (Table 1).
Characteristics of the study population.
| All population (n=100) | Manual drainage (n=55) | Vacuum bottle drainage (n=45) | p value | |
|---|---|---|---|---|
| Age, median (range), yearsa | 59 (27–91) | 59 (27–88) | 60.5 (35–91) | 0.54 |
| Etiology, n (%)a | 0.075 | |||
| Malignant | ||||
| Breast | 21 (24.13) | 11 (12.6) | 10 (11.5) | |
| Lung | 17 (19.54) | 9 (10.3) | 8 (9.19) | |
| Lymphoma | 7 (8.04) | 6 (6.89) | 1 (1.14) | |
| Kidney | 6 (6.89) | 2 (2.29) | 4 (4.59) | |
| Sarcoma | 5 (5.74) | 4 (4.59) | 1 (1.14) | |
| Gastrointestinal | 5 (5.74) | 2 (2.29) | 3 (3.44) | |
| Mesothelioma | 2 (2.29) | 1 (1.14) | 1 (1.14) | |
| Others | 13 (14.94) | 7 (8.04) | 6 (6.89) | |
| Non-malignant | ||||
| Hepatic hydrothorax | 5 (5.74) | 5 (5.74) | 0 (0) | |
| Chronic kidney failure | 4 (4.59) | 3 (3.44) | 1 (1.14) | |
| Heart failure | 1 (1.14) | 1 (1.14) | 0 (0) | |
| Idiopathic | 1 (1.14) | 0 (0) | 1 (1.14) | |
| Baseline performance status | ||||
| ECOG grade, median (range) | 2 (0–4) | 3 (1–4) | 2 (0–4) | 0.062 |
| Karnofsky score, median % (range) | 60 (20–100) | 60 (20–80) | 70 (20–100) | 0.004 |
| One-month performance status | ||||
| ECOG grade, median (range) | 1 (0–4) | 1 (0–4) | 1 (0–4) | 0.98/<0.001 |
| Karnofsky score, median % (range) | 80 (10–100) | 80 (10–100) | 80 (10–100) | 0.121/<0.001 |
| Spontaneous pleurodesis | ||||
| Yes, n (%) | 47 (47) | 26 (47.27) | 21 (46.66) | 0.75 |
| No, n (%) | 53 (53) | 29 (52.72) | 24 (53.33) | |
| Time to pleurodesis, median (range), days | 54 (8–495) | 60 (8–495) | 45 (12–159) | 0.148 |
At the time of IPC insertion, the median ECOG performance status grade was 2 (range 0–4) and the Karnofsky score 60% (range 20–100%). One month after IPC placement, significant improvements were observed in both the ECOG stage (1, range 0–4) and Karnofsky score (80%, range 10–100%) (both p<0.001). The impact of IPC on performance status at one month was independent of the drainage modality used, whether vacuum bottle or manual aspiration (p<0.98) (Table 1). No further pleural procedures were necessary in 98% of the patients after a median follow-up of 95 days (range 8–540). SP occurred in 47 cases (47%) after a median time of 54 days (range 8–495), and it was not influenced by the IPC drainage modality (p=0.773) (Table 1). The total drainage volume for patients who achieved SP using vacuum bottles was 5.83L (range 0.96–29) whereas for those who were drained manually it was 6L (range 0.4–35.7) (p=0.435).
There was no relationship between the TUS pleural fluid pattern and the development of SP (p=0.266). Ultrasonographic pleural thickening of >1cm, which was identified in 56 cases, did not have an effect on SP (p=0.071).
DiscussionThis is the first study to demonstrate that manual drainage of IPC devices is a valid and less expensive alternative to the use of vacuum bottles in low-income countries. For important patient-related outcome measures, such as the performance status or the time that IPC needs to be in place (linked to SP),7,8 no differences were found between classical vacuum bottle drainages and manual fluid aspirations. In other words, having few economic resources available should not be a barrier to palliative treatment with an IPC in patients with symptomatic persistent MPEs.
This study, however, should be considered preliminary due to its intrinsic limitations. It included a limited number of patients, though they were recruited prospectively and assigned to the drainage method according to their financial resources, thus reflecting a real-world scenario. In addition, it should be noted that IPC and talc pleurodesis are not mutually exclusive. It is plausible that in the future the combination of IPC insertion, talc slurry pleurodesis and daily catheter drainage will provide a highly cost-effective approach to symptomatic MPEs.9 Therefore, the economic impact of this or other new approaches in the removal of pleural fluid will need to be reassessed.