This study was undertaken to assess the antifertility effect of hydroalcoholic leaves extract of Pandanus odoratissimus Linn. which is traditionally used by the woman in Rajasthan state of India to regulate the fertility. The antifertility activity of the extract at dose levels (200 and 400mg/kg, orally) was evaluated in two experimental animal models. The extract was found to be safe up to a dose of 4000mg/kg of the extract when administered orally. A good antiimplantation (37.13%) activity in female rats was observed at the tested dose level (400mg/kg). The extract, when administered alone at 200mg/kg dose to immature female albino rats, enhanced the estrogen level in the serum whereas significantly decreased the estrogen level at 400mg/kg dose. The extract along with estradiol at dose level of 400mg/kg significantly (p<0.01) decreased the level of estrogen, in comparison to standard group rats indicating the antiestrogenic nature of the extract. The antiestrogenic effect of the extract at higher dose (400mg/kg) might be due to negative feed-back inhibition on anterior pituitary. Preliminary phytochemical screening has revealed the presence of alkaloids, carbohydrates, flavonoids and saponins in the hydroalcoholic leaf extract of the plant. The antifertility effect of the plant might be due to antiimplantation as well as antiestrogenic effect of the extract which in turn might be due to some of the chemical constituents present in the extract. The results shows that hydroalcoholic extract of P. odoratissimus L. leaves possess significant antifertility activity at 400mg/kg, thus, justifying the traditional use of this plant in fertility regulation in females.
The increasing population is one of the biggest problems faced by most of the countries, with its inevitable consequences on all aspects of development, especially employment, education, housing, health care, sanitation and environment.1 The whole world is now recognizing the need for fertility planning. Fertility regulation with plants or plant preparations and medicaments has been mentioned in the ancient texts of indigenous systems of medicine of many countries.2–4 The use of plants as abortifacients, emmenagogues, and local contraceptives in different countries of the world has been comprehensively summarized recently.5 The synthetic contraceptive agents currently available produce the side effects like hormonal imbalance, hypertension, increased risk of cancer and weight gain.6 Therefore, the search for safe, effective and orally active plant based alternative is highly desired for fertility regulation.
Pandanus odoratissimus L. synonym P. tectorius belonging to family Pandanaceae, is commonly known as ‘Umbrella tree’ in English and ‘Kewra’ in Hindi and is widely distributed along Indo-Malayan coasts from India and Sri Lanka throughout Southeast Asia to Taiwan, the Ryukyu Islands, Malaysian islands and Australia.7 In Ayurveda, Unani, and Siddha systems of medicine, the leaves are used for treating backache, rheumatic diseases, epilepsy, wound healing, nervous disorders, loss of appetite, indigestion, constipation, diabetes, infertility, skin diseases, urinary disorders, and fever.8
The flowers of P. odoratissimus L. are powdered and included in medicines, which are either sniffed like snuff or smoked for asthma and other bronchial infections.9 The leaves are thought to be useful in leprosy, smallpox, scabies and diseases of the heart and brain.10 The plant has been traditionally used as abortifacient by the tribal women in Rajasthan state of India.11 To the best of our knowledge, no study on the P. odoratissimus in the fertility regulation has been reported till date. Based on this evidence, we investigated the effect of the hydroalcoholic leaf extract of P. odoratissimus on fertility in female rats.
Material and methodsProcurement and identification of plant materialThe leaves of plant were collected from the botanical garden of Kurukshetra University, Kurukshetra during October 2009 and identified as P. odoratissimus L. (Family: Pandanaceae) by Dr. H.B. Singh, Scientist Incharge, Raw Materials and Museum, National Institute of Science Communication And Information Resources, New Delhi where a voucher specimen (NISCAIR/RHMD/Consult/-2009-2010/1381/183) has been deposited for further reference.
Preparation of extractAbout 620g of shade dried leaves were powdered, sieved and extracted hydro-alcohol (Ethanol: Water; 30:70) using Soxhlet at a temperature of 50°C for 72h) using soxhlet apparatus. The extract was concentrated to semisolid mass using rotary evaporator (Heidolph 4011, USA) and then lyophilized. The yield of the lyophilized extract was approximately 22.69% w/w and preserved in refrigerator at 4°C for further use.
Phytochemical screeningPreliminary phytochemical screening of the hydroalchoholic leaf extract was performed as per the reported methods to reveal the presence of major class of phytochemical constituents.12
AnimalsColony-bred healthy fertile male and female albino rats in the weight range of 150–200g were selected for the antiimplantation study whereas immature female rats in the weight range of 25–40g were used for estrogenic/antiestrogenic effect. The animals were obtained from animal house of Kurukshetra University and maintained under laboratory conditions of temperature (21.5±22°C), humidity (60±1%) and 12-h light and dark cycle. They were allowed free access to feed and water ad libitum. Experimental protocol and procedure used in the study were approved by Institutional Animal Ethical Committee (Regn. No. 562/GO/02/a/CPCSEA) of Kurukshetra University, Kurukshetra and according to the guidelines of CPCSEA, Ministry of Environment, Govt. of India, New Delhi.
Preparation of test samples and dosingThe dose of leaf extract was selected based on the previous report13 and was administered at 200 and 400mg/kg doses in the present study. The dose of extract was reconstituted by suspending the required quantity of HAEPO in Tween 80 (2% v/v in saline) freshly before use and was injected per orally (p.o.). Vehicle control groups received equal volume of Tween 80 (2% v/v in saline).
Antifertility studiesAnti-implantation activityThe fertile female rats were kept with male rats of proven fertility in the ratio of 2:1 during the proestrous or estrous phase and examined for the evidence of copulation next day morning. The rats showing the copulation plug or thick clumps of spermatozoa in their vaginal smears were separated and that day was marked as 1st day of pregnancy, and such rats were divided into three groups. Group I served as control and received only vehicle (Tween 80, 2% w/w in saline). Group II and III rats received the extract orally at the dose level of 200 and 400mg/kg from day 1 to day 7 of pregnancy. On 10th day, laparotomy was performed under light ether anesthesia using sterile conditions and uteri were examined to count the number of implantation sites.14
Esteogenic/antiesterogenic activityImmature female rats (25–45g) were divided into six groups consisting of 5 animals each. Group I (Control) was administered with vehicle (Tween 80, 2%, v/v) only. Group II (Standard) received standard drug 17α-ethinylestradiol (EE; 1μm/rat/day) suspended in olive oil subcutaneously. Group III and IV received the extract HAEPO alone at doses of 200mg/kg and 400mg/kg p.o., respectively. Group V and VI received the extract HAEPO orally along with EE (1μm/rat/day) subcutaneously for 7 consecutive days. On 8th day, all rats were sacrificed under light anesthesia. The blood serum was processed for the estimation of important biochemical parameter, i.e. estrogen level.15
Statistical analysisAll the values were expressed as mean±S.E.M. The data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's test. The levels of significance were taken at p<0.01 in relation to control and standard.
ResultsPhytochemical screeningPreliminary phytochemical studies of the extract revealed the presence of alkaloids, carbohydrates, flavonoids and saponins.
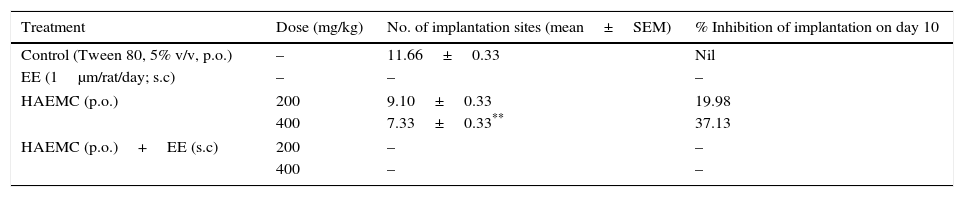
Antifertility studyAnti-implantation activityA dose dependant antiimplantation effect was observed (Table 1). With increase in the dose of the HAEPO, the percentage of implantation inhibition increased and was significant (p<0.01) at higher dose, i.e. 400mg/kg. In this study, the extract showed 19.98 and 37.13% anti-implantation effect at the doses of 200 and 400mg/kg body weight, respectively. The extract showed maximum inhibition of implants at higher dose, i.e. 400mg/kg.
Effect of HAEPO on number of implantation in female rats.
| Treatment | Dose (mg/kg) | No. of implantation sites (mean±SEM) | % Inhibition of implantation on day 10 |
|---|---|---|---|
| Control (Tween 80, 5% v/v, p.o.) | – | 11.66±0.33 | Nil |
| EE (1μm/rat/day; s.c) | – | – | – |
| HAEMC (p.o.) | 200 | 9.10±0.33 | 19.98 |
| 400 | 7.33±0.33** | 37.13 | |
| HAEMC (p.o.)+EE (s.c) | 200 | – | – |
| 400 | – | – | |
N=6; Nil=zero.
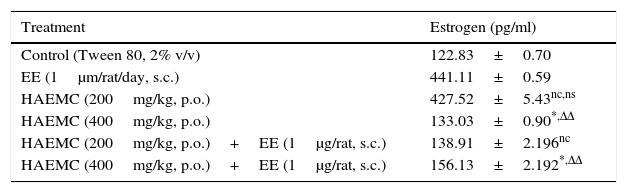
The effect of extract on various biochemical parameter has been shown in Table 2. The HAEPO extract, when administered alone at 200mg/kg dose to immature female albino rats, enhanced the estrogen level in the serum whereas decreased the estrogen level at 400mg/kg dose. The extract along with estradiol at dose level of 400mg/kg significantly (p<0.01) decreased the level of estrogen, in comparison to standard group rats indicating the antiestrogenic nature of the extract.
Effect of HAEMC on biochemical parameter in immature ovariectomized female rats.
| Treatment | Estrogen (pg/ml) |
|---|---|
| Control (Tween 80, 2% v/v) | 122.83±0.70 |
| EE (1μm/rat/day, s.c.) | 441.11±0.59 |
| HAEMC (200mg/kg, p.o.) | 427.52±5.43nc,ns |
| HAEMC (400mg/kg, p.o.) | 133.03±0.90*,ΔΔ |
| HAEMC (200mg/kg, p.o.)+EE (1μg/rat, s.c.) | 138.91±2.196nc |
| HAEMC (400mg/kg, p.o.)+EE (1μg/rat, s.c.) | 156.13±2.192*,ΔΔ |
N=6; nc – not significant with respect to control: p>0.05; ns – not significant with respect to standard; * – significant with respect to control: p<0.01; ΔΔ – significant with respect to standard: p<0.01.
In spite of the widespread use of herbal medicines, few scientific studies have been undertaken to ascertain the possible mechanism of action and efficacy of traditional remedies.16 Since P. odoratissimus L. has been traditionally used to induce abortion among the women in Rajasthan, India11 and no scientific evidence in this regard is present till date, the present study was performed to evaluate the anti-fertility effect of hydroalcholic leaves extract (HAEPO) of this plant in female rats. The extractive values of hydroalcoholic leaves extracts obtained as 22.692% (w/w), respectively, which gives an idea about the solubility pattern of the phytoconstituents present in the drug.
In anti-implantation study, a dose dependant anti-implantation effect was observed (Table 1). With increase in the dose of the HAEPO, the percentage of implantation inhibition increased and was significant (p<0.01) at higher dose, i.e. 400mg/kg. In this study, the extract showed 19.98 and 37.13% anti-implantation effect at the doses of 200 and 400mg/kg body weight, respectively. The extract showed maximum inhibition of implants at higher dose, i.e. 400mg/kg.
The HAEPO extract, when administered alone at 200mg/kg dose to immature female albino rats, enhanced the estrogen level in the serum whereas decreased the estrogen level at 400mg/kg dose. The extract along with estradiol at dose level of 400mg/kg significantly (p<0.01) decreased the level of estrogen, in comparison to standard group rats indicating the antiestrogenic nature of the extract.
The antiestrogenic effect of the HAEPO extract at higher doses (400mg/kg) might be due to negative feed-back inhibition on anterior pituitary. Both estrogenic and anti-estrogenic properties have also been shown by the other plants in previous studies.17–20 For the implantation and sustenance of pregnancy, exact equilibrium of secretion of estrogen and progesterone is necessary. Any imbalance in these hormones may cause anti-implantation or induce abortion.21 The compounds of hormonal value usually disturb the hormonal milieu in the uterus and provoke an anti-fertility effect.22 It has already been reported that steroids,23 flavonoids (flavones, flavonones and isoflavones) alkaloids and phenolics occurring in variety of plants24,25 are reported to have shown anti-fertility activity in laboratory animals. Preliminary phytochemical screening has revealed the presence of alkaloids, carbohydrates, flavonoids and saponins in the hydroalcoholic leaf extract of P. odoratissimus L. Therefore, the presence of one of these phytoconstituents might be responsible for the anti-fertility activity of hydroalcoholic leaves extract of P. odoratissimus.
ConclusionFrom the present study, it concluded that hydroalcoholic leaves extract of P. odoratissimus leaves possesses significant antifertility effect which might be due to the inhibition of implantation and antiestrogenic effect which in turn might be due to the presence some phytoconstituents in the plant. Further studies are required to elucidate exact mechanism of antifertility action and isolation of the active components responsible for antifertility effect.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are thankful to Director, Institute of Pharmaceutical Sciences, Kurukshetra University, Kurukshetra for providing necessary support and facilities during the study.