The aim of this study is to evaluate if the extension of neoadjuvant endocrine therapy (NET), beyond the conventional time, allows additional downstage of the tumour, in order to perform a breast conservative surgery (BCS), and to analyze if it is a good option for long-term control in patients who refuse or are unfit for surgery.
Patients and methodsWe retrospectively reviewed a database containing all patients treated in our institution with NET. All included patients were post-menopausal with primary local disease. The type of response obtained was assessed using modified RECIST criteria.
ResultsThirty-three patients were included. Two patients had tumours with 90% expression of oestrogen receptors and all the others had 100%. The tumour size in the largest diameter was 6.51cm before treatment and 5.18cm after. Eighteen patients achieved a partial response after 10.28 months of therapy. Patients that were proposed to downstage the tumour performed 9.71 months of therapy until surgery and all were submitted to BCS. Progression occurred after 27.5 months.
ConclusionEndocrine therapy is a feasible option for a longer time to allow additional downstage of the tumour and is a good solution in patients who refuse or are unfit for surgery.
Breast cancer (BC) is the most common type of cancer in women.1,2 Its incidence is expected to increase with ageing of the population.1,2 As estrogens have a major impact in BC development and progression, endocrine therapy is an increasingly used treatment option both as adjuvant (after surgery) or neoadjuvant (alone or before surgery).3–5 Currently used drugs are tamoxifen and aromatase inhibitors (AIs): letrozole, anastrozole and exemestane.
In what concerns pre-menopausal women, neoadjuvant endocrine therapy (NET) is contra-indicated3,6,7 because studies are lacking to take conclusions, and for the moment, the ones that exist are contradictory.8,9 On the other hand, regarding post-menopausal patients, in the most important international Guidelines3,6,7,10 there are no precise orientations related to NET.
For many years tamoxifen was validated as initial sole treatment for frail elderly women, with overall response rates achieving 73%.11 A study from Nottingham randomized women with estrogen receptor (ER)-positive cancers for mastectomy plus 5 years of tamoxifen versus tamoxifen alone for 5 years.12 The update of the phase III GRETA trial did the same but there were no data concerning ER expression.13 Some results of the studies mentioned are shown in Table 1.
Nevertheless, the group in which there is more controversy is that of young and fit postmenopausal women with inoperable locally advanced tumour in whom breast conservative surgery is not possible.3,9,14–18 In luminal cancers, the pathologic complete response (pCR) rate to chemotherapy is much lower and, consequently, neoadjuvant chemotherapy is of limited benefit.3,9,14–17 Actually, there are some studies that compare hormonal therapy to chemotherapy before surgical treatment in strong ER expression tumours.9,15,19 The major results of those studies are shown in Table 2.
Besides the controversy over the effectiveness of NET, there is also no consensus on how long the treatment should last. The conventional treatment period is 3–4 months.16,17,20,21 However, recent trials have reported better results with the extension of the treatment's duration.17,18
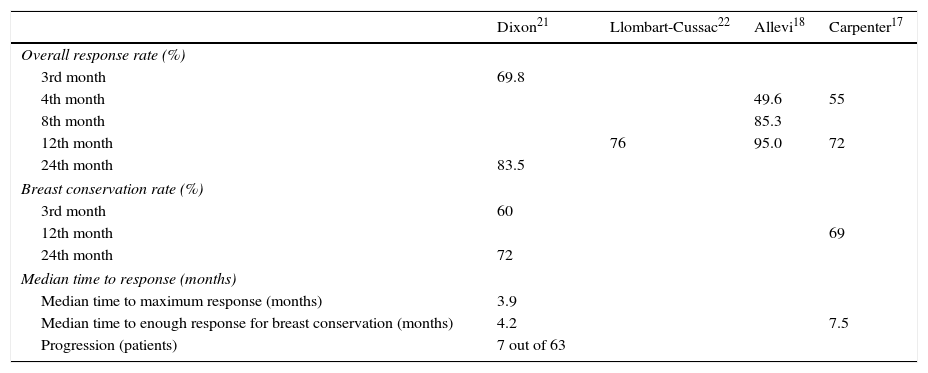
Dixon et al. conducted a study in which patients with locally advanced ER-positive cancers were submitted to neoadjuvant letrozole.21 More recently Llombart-Cussac et al. published a small phase II trial with letrozole and the goal was to establish mean time to maximum response.22 Until the 4th month those who progressed or had stable disease were excluded, 37.1% women improved after the 6th month but none after the 8th month.22 In 2013, Allevi et al. created three cohorts with 40 patients each, that took letrozole for 4, 8 and 12 months.18 All women were older than 65 years old, with luminal BC and unfit for chemotherapy.18 Carpenter et al., in 2014, conducted a study with post-menopausal women not eligible for breast conservation, that took letrozole for 12 months.17 The most important results of the studies mentioned above are described in Table 3.
Results of the studies of Dixon, Llombart-Cussac, Allevi and Carpenter.
| Dixon21 | Llombart-Cussac22 | Allevi18 | Carpenter17 | |
|---|---|---|---|---|
| Overall response rate (%) | ||||
| 3rd month | 69.8 | |||
| 4th month | 49.6 | 55 | ||
| 8th month | 85.3 | |||
| 12th month | 76 | 95.0 | 72 | |
| 24th month | 83.5 | |||
| Breast conservation rate (%) | ||||
| 3rd month | 60 | |||
| 12th month | 69 | |||
| 24th month | 72 | |||
| Median time to response (months) | ||||
| Median time to maximum response (months) | 3.9 | |||
| Median time to enough response for breast conservation (months) | 4.2 | 7.5 | ||
| Progression (patients) | 7 out of 63 | |||
Given that there are no standardized guidelines on how long NET should be performed, there is a real need for more studies focusing on this matter. Therefore, the aim of this study is to evaluate whether the extension of NET, beyond the conventional time of3–4 months, allows additional downstage of the tumour, in order to perform a breast conservative surgery (BCS), and to analyze if it is a good option for long-term control in patients who refuse or are unfit for surgery.
Patients and methodsWe retrospectively reviewed a database containing all patients with BC, from January 2007 until October 2015, who were treated with NET (with or without surgery) in our institution. All included patients were post-menopausal women with primary local disease, who had been submitted to NET with tamoxifen or AIs. Patients excluded were those with metastatic disease, inflammatory BC, previous radiation directed to the chest or chemotherapy for other concurrent cancer during the period of study.
We collected data concerning age; indication to endocrine therapy; multifocality; tumour size (T) and status of axillary nodes (N), according to TNM staging system for BC; tumour histologic subtype and grade; status and percentage of expression of ER and progesterone receptors (PR); HER2Neu amplification; drug used; response to treatment; time to response and surgery. Positive ER expression is defined as >1% of expression and positive PR expression as >20% of expression.
Tumour size before treatment was assessed through mammography and ultrasound in all patients. Post-treatment size was measured in the surgical specimen in one patient and obtained through clinical observation in two patients; all the others were measured by mammography and ultrasound. The efficacy of the treatment was evaluated using modified RECIST criteria: (1) total response: the tumour is no longer palpable or visible in images; (2) partial response: sustained reduction of at least 30% of the tumour size; (3) progression: an increase of 20% or a new lesion, axillary node or metastasis; and (4) partial response followed by progression: reduction of at least 30% of the tumour size followed by an increase of 20% or a new lesion, axillary node or metastasis.2,23
Immunohistochemistry was carried out to assess: HER2 status, using the anti-Her-2/neu (4B5) rabbit monoclonal antibody; ER, with the anti-estrogen receptor (SP1); PR, with the anti-progesterone receptor (1E2); using the Bench Mark ULTRA. Number of copies of the HER2/neu gene was obtained through Silver-enhanced in situ hybridization (SISH), using the BenchMark XT and the probe INFORM TM. The equipment is commercialized by Ventana® Medical Systems (Tucson, EUA).
Statistical analysis was performed using the SPSS® software version 23.0 (SPSS Inc., Chicago, IL). For categorical variables data was presented as absolute frequencies and percentages. For continuous variables, data was presented as mean±standard deviation. Categorical variables were compared using chi-squared test while continuous variables were compared using independent samples t-test. The size of the tumour before and after treatment was analyzed using a Wilcoxon Test. Differences were considered statistically significant if the corresponding p-value was ≤0.05.
The study was approved by the Institutional Review Board and thus meets the standards of the Declaration of Helsinki.
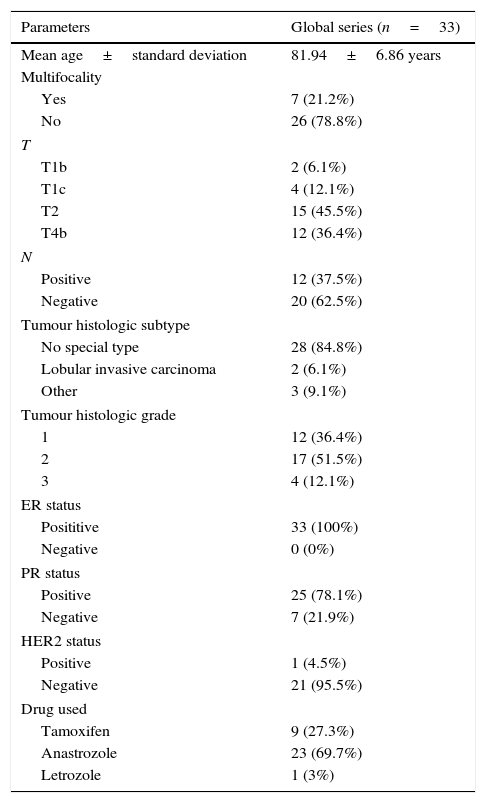
ResultsThirty-three patients were included. The mean age was 81.94±6.86 years old, with a maximum of 97 years old. Twelve (36.4%) of our patients had tumours ulcerating the skin. Two patients had tumours with 90% expression of ER and all the others had 100%. In our institution, HER2 expression/amplification is not routinely determined in the elderly. The baseline characteristics of our population are shown in Table 4.
Baseline characteristics of the population included in this study.
| Parameters | Global series (n=33) |
|---|---|
| Mean age±standard deviation | 81.94±6.86 years |
| Multifocality | |
| Yes | 7 (21.2%) |
| No | 26 (78.8%) |
| T | |
| T1b | 2 (6.1%) |
| T1c | 4 (12.1%) |
| T2 | 15 (45.5%) |
| T4b | 12 (36.4%) |
| N | |
| Positive | 12 (37.5%) |
| Negative | 20 (62.5%) |
| Tumour histologic subtype | |
| No special type | 28 (84.8%) |
| Lobular invasive carcinoma | 2 (6.1%) |
| Other | 3 (9.1%) |
| Tumour histologic grade | |
| 1 | 12 (36.4%) |
| 2 | 17 (51.5%) |
| 3 | 4 (12.1%) |
| ER status | |
| Posititive | 33 (100%) |
| Negative | 0 (0%) |
| PR status | |
| Positive | 25 (78.1%) |
| Negative | 7 (21.9%) |
| HER2 status | |
| Positive | 1 (4.5%) |
| Negative | 21 (95.5%) |
| Drug used | |
| Tamoxifen | 9 (27.3%) |
| Anastrozole | 23 (69.7%) |
| Letrozole | 1 (3%) |
Patients were submitted to NET due to comorbidities in 13 patients (39.4%) and refusal to undergo surgery in 12 (36.4%). Eight patients (24.2%) had the referred treatment to downstage the tumour prior to surgery.
Thirteen surgeries were performed: 8 BCS that represent all the patients in the group that was doing NET to downstage the tumour; 4 surgeries after progression on endocrine therapy (2 mastectomies and 2 BCS); 1 mastectomy in 1 patient unable to undergo surgery from the start for psychological reasons.
The tumour size in the largest diameter was 6.51±11.00cm before and 5.18±10.28cm after treatment (p=0.008), which represents a statistically significant reduction in tumour size.
Eighteen patients (54.5%) achieved a partial response; 4 (12.1%) showed partial response followed by progression. Stable disease was observed in 7 (21.2%) of the patients and 4 (12.1%) suffered progression. No patient showed complete clinical response nor pCR.
The mean time to obtain response in the group with partial response was 10.28±6.85 months, with a minimum of 3 and a maximum of 24 months. 11.11% of the patients (2 in 18) achieved response at the 4th month or earlier and 38.89% (7 in 18) achieved response in the 6th month or earlier. In the group with stable disease, the time elapsed from the beginning of therapy until last visit was 11.57±7.70 months.
In the patients with partial response followed by progression, the time during which response was documented was 13.50±3.42 months; after that time, the women remained stable until progression occurred in the 22nd month or later and, in average, after 35.25±13.37 months of therapy, which means that after a reduction in tumour size have ceased to be observed, 21.75 months of stability went by until the disease progressed. Patients who suffered progression without any previous response, did so only after 19.75±8.01 months of treatment. The overall mean time to progression (with or without initial partial response) was 27.5±13.47 months.
Analyzing those patients that refused or had co-morbidities to undergo surgery, we found that overall progression occurred in 32% (8 in 25) of the cases but only one women who progressed did it before the first year of treatment (8th month); actually, four patients progressed between the 18th and 24th month and another three between the 40th and 58th month, which was the maximum. The patients proposed to downstage the tumour had performed 9.71±6.84 months of therapy until surgery.
There were no statistically significant differences when comparing patients’ and tumours’ characteristics and the type of response using modified RECIST criteria.
DiscussionNowadays, endocrine therapy as sole treatment or before surgery is generally reserved for frail elderly women, patients with comorbidities regardless of age and women who refuse surgery. Not surprisingly, mean age was 81.94 years old and the indication for endocrine therapy in 76% of our patients was co-morbidities or surgery refusal.
Incoming with previous studies, our results also demonstrate that endocrine therapy is effective – tumour size in the largest diameter was 6.51cm before and 5.18cm after treatment, representing a statistically significant reduction in tumour size. Therefore, this reinforces that NET is a good alternative to chemotherapy for Luminal tumours.9,15,19
In our literature review, the overall response rate to NET (with or without surgery), regarding women with strong ER expression, ranged from 58 to 83.5%.8,9,15,18,19,22 Since our patients did not achieve complete clinical response, our overall response rate equals the rate of partial response, which was 54.55%. We believe this slightly difference is related to two facts: 9 patients (27.3%) were on tamoxifen, that has been shown to perform worse than aromatase inhibitors,8,18,24,25 and not all of our patients are Luminal A breast cancers – HER2 expression/amplification was determined only in 22 patients (66.7%), 4 tumours (12.1%) were grade 3 and 7 (21.9%) did not express progesterone receptors.
In our study, we did not determine the pre-treatment eligibility to BCS. However, all the eight patients proposed to tumour's downstage, after 9.71 months of NET therapy, preserved their breast, which supports recent trials that suggested that the extension of endocrine therapy would improve maximum reduction in tumour volume, sufficient for BCS.17,20,21
Llombart-Cussac et al. suggested endocrine resistance if the disease is stable or if it is progressed by the 4th month.22 On the other hand, if at this time there is any response, treatment duration should be individualized.22 The median time to achieve clinical response, in the 18 patients who had partial response was 10.28 months. Moreover, on that group, only 11.11% of patients (2 in 18) achieved response at the 4th month or earlier and 38.89% (7 in 18) achieved response at the 6th month or earlier. Therefore, in our study, the required time of therapy to suggest endocrine resistance is longer than the 4 months suggested by Llombart-Cussac et al.22
Some tumours, despite having ER expression, fail to respond.26 45.4% of the patients (the groups of stable disease, progression and partial response followed by progression) exhibited resistance to endocrine therapy. According to Carpenter et al., the neoadjuvant approach allows early selection of endocrine resistant ER-positive tumours.17
In previous studies, pCR rate ranges from 0% to 3%,8,9,15,19,22 except in one study by Allevi et al. that reaches 17.5% by 12 months, although we did not find any explanation for this result.18 In our study, the pCR rate was 0%. However, unlike neoadjuvant chemotherapy, pCR in the context of NET is not considered prognostic.16
In GRETA trial, even in those patients that took tamoxifen alone, median time for progression was 19.3 months and if, while waiting for maximum response, progression occurred, this delay did not worsened prognosis.13 In fact, our results show that patients who suffered progression (with or without partial response), progressed after a mean time of 27.5 months. During that time those patients remained with stable disease or had partial response. That represents that even patients who had the worst outcome only began to suffer latter in the course of therapy. In the case of patients with a low life expectancy and who refused to or cannot undergo surgery, it is a long time of disease control.
Our study has some limitations: being retrospective we have not programmed follow-up visits, therefore we were not able to determine maximal time to response; also 9.1% (3 in 33) of our patients were not measured by ultrasound or mammography after treatment, as it is recommended by the RECIST criteria. Thirty-three is a small number of patients, however, it represents the elderly patients treated with NET, in our institution, in the last 9 years. Finally, we did not evaluate functional status of our patients.
In conclusion, our study support that NET can be done beyond the conventional3–4 months to allow additional downstage of the tumour and, therefore, preserve the breast,17,18 because all patients proposed to downstage, after 9.71 months of NET, were submitted to BCS. Besides that, in the group which achieved response, 10.28 months was the mean time to accomplish it, also a longer time than the conventional. Our results showed that even patients who had the worst outcome, this is, who progressed, only began to suffer latter in the course of therapy, after a mean time of 27.5 months, which in the case of patients with a low life expectancy and who refused to or cannot undergo surgery, represents a long time of disease control.
Conflicts of interestThe authors declare no conflicts of interest.