Community-acquired pneumonia (CAP) is a frequent cause of admission to hospital worldwide with high mortality rates. Host comorbidities may be associated not just with a greater risk of developing the disease but also with worse outcomes. In this work, the evaluation of the impact of host comorbidities on the prognosis of severe CAP patients admitted to an Intensive Care Unit (ICU) was proposed. Severity indexes, some clinical and analytic parameters at admission in ICU as well as patient comorbidities were analyzed and statistically compared with mortality. In this study, although there was no clear link between comorbidities and mortality, factors such as smoking, obesity and previous renal disease impairment seem to have an impact on the prognosis of severe CAP.
Community-acquired pneumonia (CAP) is a common illness with an overall rate in adults of approximately 5.16–6.11 cases per 1000 persons per year.1 It is one of the main causes of morbidity and mortality worldwide.2–4 In developed countries, CAP is the first infectious cause of mortality with about 28% mortality within one year.5,6 CAP is also the infectious disease with the highest health costs, as up to a third of patients needs to be admitted to hospital.2,7 In Portugal, the hospital admission rate by CAP represented 3.7% of the total number of admissions between 2000 and 2009 with a mortality rate of 20.4%.8 Previous studies have shown that approximately 18% of patients admitted to hospital matched the criteria for severe CAP and mortality seems to be higher in these patients.5,9 Pneumonia incidence and severity of disease are increased in the elderly5,10,11 which could be explained by aging of organ systems and the presence of comorbidities.10–12 CAP is more common in men and in black people and there is a seasonal variation, with more cases occurring during the winter months.1
Concerning lower respiratory infections, three entities should be differentiated. CAP is an infection of the pulmonary parenchyma caused by an agent acquired in the community and should be distinguished from nosocomial or hospital acquired pneumonia (HAP) which develops at least 48h after hospital admission, or from health-care associated pneumonia (HCAP), which occurs in patients which have been admitted to hospital during the preceding 90 days, receiving dialytic treatment during the preceding 30 days, residing in a nursing home, using home intravenous treatment or home wound care or having close contact with a person harboring multi-drug resistant (MDR) pathogens.3,13,14 These last two entities have a higher risk for MDR agent infection.1
Severe infection of the pulmonary parenchyma is the most frequent risk factor for acute respiratory distress syndrome (ARDS).5 ARDS is defined by the acute onset of respiratory failure within 1 week of a clinical insult, bilateral opacities consistent with pulmonary edema on chest radiograph or computed tomography, hypoxemia with a PaO2/FiO2 ratio ≤300mmHg on a minimum positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) of 5cmH2O and no objective evidence of cardiac failure or fluid overload. ARDS is categorized as mild (PaO2/FiO2 [200–300]), moderate (PaO2/FiO2 [100–200]) or severe (PaO2/FiO2<100), according to the grade of hypoxemia.15 Age and factors associated with clinical disorders may have an impact on the incidence of ARDS.16–18 ARDS is associated with appreciable mortality, with estimates ranging from 26 to 58%, and is one of the main reasons for hospital admission.5,7 CAP is the most common focus of infection leading to severe sepsis.19 Sepsis is defined as a life-threatening organ dysfunction caused by a deregulated host response to infection. Sepsis can evolve to septic shock with an even higher risk of death.20
CAP is more frequently caused by virus and bacteria although fungi and parasites can also be etiologic agents in some contexts. However, in many cases of diagnosed CAP based in clinical and radiologic findings, the etiology cannot be defined.3,12 Human rhinovirus, Influenza virus and Streptococcus pneumoniae are the most commonly detected pathogens.1,3 The indirect protection of adults as a result of pediatric and adult pneumococcal vaccination may potentially contribute to a slow decrease in the incidence of pneumococcal infection, but for the time being data is missing in our country.1,3,21Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella pneumophila are also common and are often referred to as “atypical” agents.12 Agents such as S. pneumoniae, Enterobacteriaceae and Staphylococcus aureus are overrepresented among severely ill patients as well as patients with associated comorbidity, prior influenza infection or antimicrobial treatment.1,3 Among patients who require admission to an ICU, S. pneumoniae is the most commonly detected pathogen.1 Also, S. pneumoniae is the most frequently causative microorganism in smokers, particularly in invasive pneumococcal disease and septic shock.22 Patients who are severely ill with influenza pneumonia should be evaluated for secondary bacterial infection, which is most likely to be caused by S. pneumoniae, group A Streptococcus and S. aureus (including community methicillin-resistant (MRSA)). C. psittaci should be considered in the case of exposure to birds. In patients who present certain comorbidities or some risk for HCAP, MRSA and multidrug-resistant gram-negative bacilli should be considered. MRSA is an important cause of severe, occasionally necrotizing CAP. Pneumocystis jirovecii is a possible agent in patients with immunodepression such as human immunodeficiency virus (HIV) infection, autoimmune diseases, transplanted or under immunosuppressive drugs. Fungal infection is an unusual cause of CAP in the immunocompetent patient, but certain fungi (e.g. Histoplasma capsulatum, Coccidioides spp.) can cause pneumonia in patients who live in or have visited endemic areas. Due to globalization, etiology of pneumonia nowadays is a dynamic issue, as evidenced by the emergence of avian influenza viruses, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS-CoV).1
Epidemiological evidence suggests that, in addition to pathogen prevalence and virulence, host related factors play a critical role in determining both susceptibility to and outcome from pulmonary infections.23,24 Many studies have sought to identify factors during the acute illness capable of predicting the outcome. Increasing age, severity of acute illness, certain pre-existing medical conditions, organ dysfunctions requiring support and emergency admission to ICU are proposed factors related to increased risk of in-hospital mortality.5,25
Curb-65 and Pneumonia Severity Index (PSI) are prognostic predicting systems for patients with CAP. They also help guiding the choice of the initial site of treatment, including ICU admission. The PSI score, although it is not a score easy to apply in the emergency department, has a higher discriminatory power for short-term mortality than CURB-65, especially for low risk patients.26 The Acute Physiologic and Chronic Health Evaluation (APACHE II), the Simplified Acute Physiology Score (SAPS II) and the Sepsis-related Organ Failure Assessment (SOFA) are used in patients admitted in the ICU. The first two scores are admission scoring systems and the last one is a repetitive scoring system. The APACHE score is probably the best-known and the most widely used score. Scoring systems essentially consist of two parts: the severity score (the higher the number, the greater the severity of the condition) and the calculation of mortality risk.25
The aim of this study is to understand the impact of comorbidities on the prognosis of severe CAP by accessing clinical and analytic parameters, severity index scores, evolution and mortality of patients admitted to the ICU of CHSJ Infectious Diseases Service from January 2013 to December 2015.
MethodsPatientsComplete electronic medical records of patients admitted by severe CAP to an ICU from January 1st 2013 to December 31st 2015 were included. Patients with HAP or HCAP were excluded.
Data collectionPrevious presence of comorbidities such as chronic diseases, noxious habits and medical immunossupression were assessed. Any documentation on medical records of HIV infection, hepatic insufficiency/cirrhosis, diabetes mellitus (DM), active malignant neoplasia, heart disease, hypertension, obesity, dyslipidemia, chronic respiratory disease, chronic kidney disease and chronic digestive disease were considered chronic diseases. Current tobacco smoking habits and high levels of alcohol consumption (>20g of alcohol per day for men or >10g of alcohol per day for women) were considered noxious habits. Current chemotherapy and immunossupressive drug treatment were considered medical immunosuppression. Patients with HIV infection, hepatic insufficiency/cirrhosis, DM and active malignant neoplasia, alcoholic patients and patients under medical immunossupression were considered immunodepressed. Clinical and analytic parameters were registered such as hematocrit (Ht), white blood cell count, platelet number (Plt), C-reactive protein (CRP), albumin (Alb), total bilirubin (Bil), urea (U), creatinine (Cr), glucose and sodium, lactate, pH, fraction of oxygen in the inspired air (FiO2), partial pressure of oxygen (PaO2) and PaO2/FiO2 ratio, respiratory rate, pulse, temperature, diastolic arterial pressure (DAP), systolic arterial pressure (SAP) and presence or absence of confusion and pleural effusion on first 24h of ICU admission. Severity indexes were calculated for each patient. Patient evolution during hospitalization was assessed by discharge results (recuperation or mortality) and by the need of support for organ dysfunctions like vasopressor support (amines administration), invasive (IMV) or non-invasive (NIV) ventilatory support, renal replacement therapy (RRT) and extracorporeal membrane oxygenation (ECMO). Clinical and analytic parameters and their limit range were selected based on established severity indexes, except for C-reactive protein, albumin and lactate.
These variables were statistically compared in order to conclude their impact on mortality.
Mortality riskCurb-65, PSI, APACHE II, SAPS II and SOFA indexes were calculated to assess the severity of pneumonia and to predict in-hospital mortality.
Statistical analysisData analysis was performed using the GraphPad Prism 7.02 software. Univariate comparisons of binary variables were conducted by means of continuity adjusted Y2-tests; for continuous variables, the Mann–Whitney nonparametric two-sample test was used as values are not normally distributed. A p-value of <0.05 was considered statistically significant. Probabilities of mortality were calculated using the Kaplan–Meier estimator for each variable and hazard ratios were calculated by the log rank method with 95% confidence intervals. Median survival was defined as the time since admission to ICU and death from any cause during the time spent in hospital. Data regarding patients who were alive at the time of the analysis were censored. The severity indexes at ICU admission were also compared using these two statistical methods. Each severity index was analyzed by score comparing ‘low-risk’ and ‘high-risk’ patients (scoring≤ vs >median severity index points) to determine median survival time and mortality risk differences.
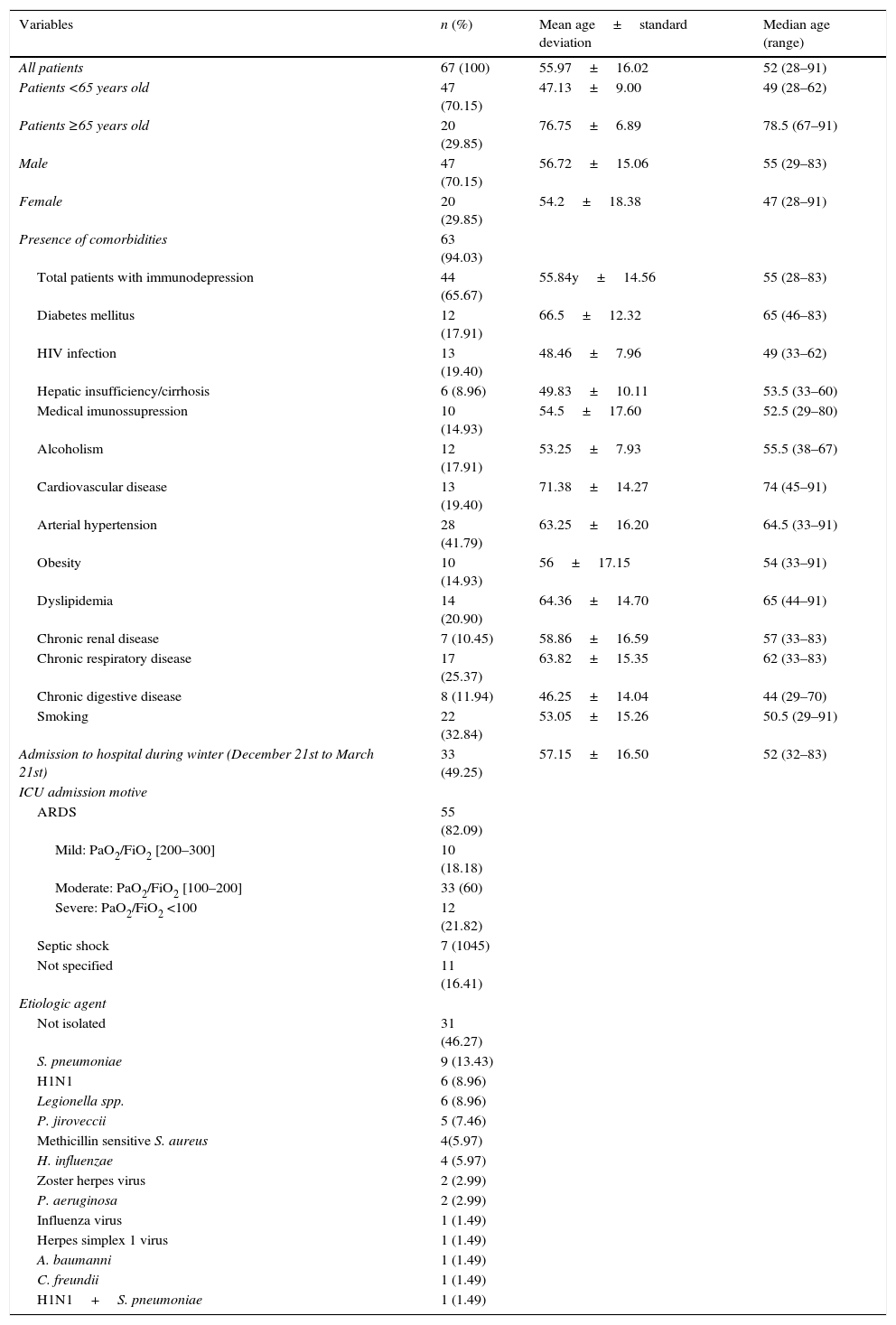
ResultsPatient characteristicsThe study included 67 patients. All patients admitted were empirically treated with ceftriaxone and azithromycin according to hospital protocol. Patient characteristics are described in Table 1. The mean age of patients is 55.97±16.02 years old, with 29.85% of the patients older than 65. The median age of patients is 52 years old. There were 29.85% women and 70.15% men. ICU admission was mainly due to respiratory insufficiency in 82.09% (18.18% mild ARDS, 60% moderate ARDS and 21.82 severe ARDS) and septic shock in 10.45% patients. One or more comorbid conditions were present in 94.03% patients. There were 65.67% immunodepressed patients and the mean age of this group was 55.84±14.56 years old. Patients with HIV infection, chronic digestive disease or smokers had mean ages under 52 years old (median age of sample). Hospital admissions during the winter (March 21st to December 21st) represented 50.75% of the cases. S. pneumoniae was isolated in 13.43% and H1N1 was present in 8.96% of the patients. Other agents were found in lesser percentages. Coinfection by more than one agent was found in one patient (1.49%). In 47.27% of the cases, the etiologic agent was not identified. The global mortality rate was 26.87%. Mortality rate for patients with ARDS was 29.09% and for those with septic shock was 42.86%.
Patient characteristics (n=67).
| Variables | n (%) | Mean age±standard deviation | Median age (range) |
|---|---|---|---|
| All patients | 67 (100) | 55.97±16.02 | 52 (28–91) |
| Patients <65 years old | 47 (70.15) | 47.13±9.00 | 49 (28–62) |
| Patients ≥65 years old | 20 (29.85) | 76.75±6.89 | 78.5 (67–91) |
| Male | 47 (70.15) | 56.72±15.06 | 55 (29–83) |
| Female | 20 (29.85) | 54.2±18.38 | 47 (28–91) |
| Presence of comorbidities | 63 (94.03) | ||
| Total patients with immunodepression | 44 (65.67) | 55.84y±14.56 | 55 (28–83) |
| Diabetes mellitus | 12 (17.91) | 66.5±12.32 | 65 (46–83) |
| HIV infection | 13 (19.40) | 48.46±7.96 | 49 (33–62) |
| Hepatic insufficiency/cirrhosis | 6 (8.96) | 49.83±10.11 | 53.5 (33–60) |
| Medical imunossupression | 10 (14.93) | 54.5±17.60 | 52.5 (29–80) |
| Alcoholism | 12 (17.91) | 53.25±7.93 | 55.5 (38–67) |
| Cardiovascular disease | 13 (19.40) | 71.38±14.27 | 74 (45–91) |
| Arterial hypertension | 28 (41.79) | 63.25±16.20 | 64.5 (33–91) |
| Obesity | 10 (14.93) | 56±17.15 | 54 (33–91) |
| Dyslipidemia | 14 (20.90) | 64.36±14.70 | 65 (44–91) |
| Chronic renal disease | 7 (10.45) | 58.86±16.59 | 57 (33–83) |
| Chronic respiratory disease | 17 (25.37) | 63.82±15.35 | 62 (33–83) |
| Chronic digestive disease | 8 (11.94) | 46.25±14.04 | 44 (29–70) |
| Smoking | 22 (32.84) | 53.05±15.26 | 50.5 (29–91) |
| Admission to hospital during winter (December 21st to March 21st) | 33 (49.25) | 57.15±16.50 | 52 (32–83) |
| ICU admission motive | |||
| ARDS | 55 (82.09) | ||
| Mild: PaO2/FiO2 [200–300] | 10 (18.18) | ||
| Moderate: PaO2/FiO2 [100–200] | 33 (60) | ||
| Severe: PaO2/FiO2 <100 | 12 (21.82) | ||
| Septic shock | 7 (1045) | ||
| Not specified | 11 (16.41) | ||
| Etiologic agent | |||
| Not isolated | 31 (46.27) | ||
| S. pneumoniae | 9 (13.43) | ||
| H1N1 | 6 (8.96) | ||
| Legionella spp. | 6 (8.96) | ||
| P. jiroveccii | 5 (7.46) | ||
| Methicillin sensitive S. aureus | 4(5.97) | ||
| H. influenzae | 4 (5.97) | ||
| Zoster herpes virus | 2 (2.99) | ||
| P. aeruginosa | 2 (2.99) | ||
| Influenza virus | 1 (1.49) | ||
| Herpes simplex 1 virus | 1 (1.49) | ||
| A. baumanni | 1 (1.49) | ||
| C. freundii | 1 (1.49) | ||
| H1N1+S. pneumoniae | 1 (1.49) | ||
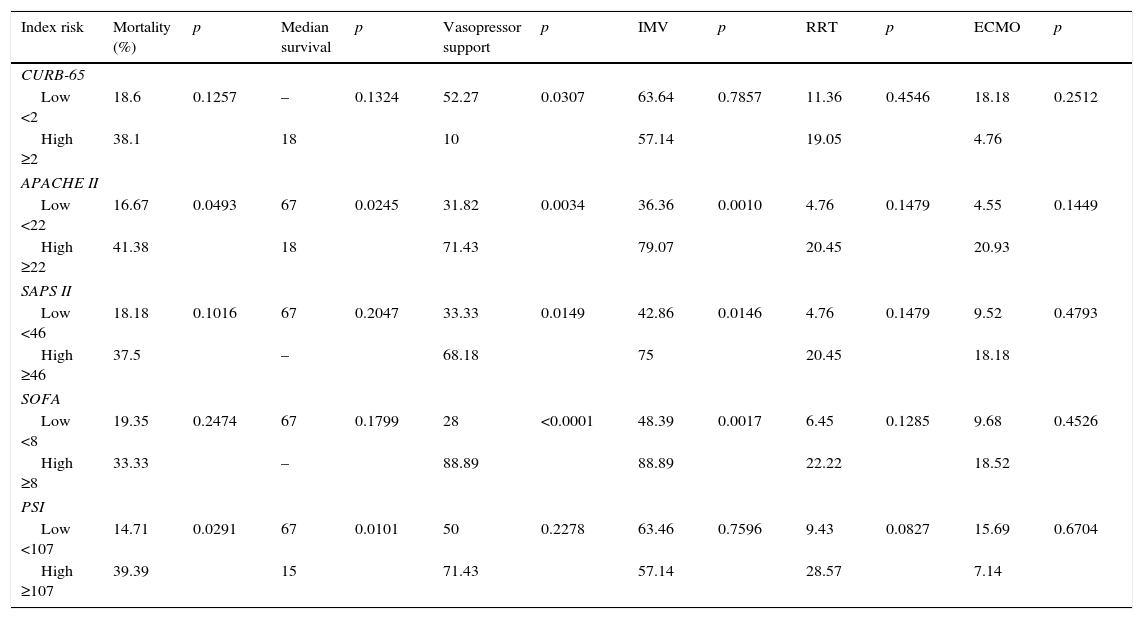
Comparison of the different severity indexes’ capacity for predicting mortality, median survival and treatment support is described in Table 2. APACHE II and PSI higher scores significantly predicted mortality (41.38%, p=0.0493 and 39.39%, p=0.0291, respectively) and these patients had a significantly lower median survival time (18 days, p=0.0245 and 15 days, p=0.0101, respectively).
Comparison of different severity indexes capacity in predicting mortality, median survival and treatment support.
| Index risk | Mortality (%) | p | Median survival | p | Vasopressor support | p | IMV | p | RRT | p | ECMO | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CURB-65 | ||||||||||||
| Low <2 | 18.6 | 0.1257 | – | 0.1324 | 52.27 | 0.0307 | 63.64 | 0.7857 | 11.36 | 0.4546 | 18.18 | 0.2512 |
| High ≥2 | 38.1 | 18 | 10 | 57.14 | 19.05 | 4.76 | ||||||
| APACHE II | ||||||||||||
| Low <22 | 16.67 | 0.0493 | 67 | 0.0245 | 31.82 | 0.0034 | 36.36 | 0.0010 | 4.76 | 0.1479 | 4.55 | 0.1449 |
| High ≥22 | 41.38 | 18 | 71.43 | 79.07 | 20.45 | 20.93 | ||||||
| SAPS II | ||||||||||||
| Low <46 | 18.18 | 0.1016 | 67 | 0.2047 | 33.33 | 0.0149 | 42.86 | 0.0146 | 4.76 | 0.1479 | 9.52 | 0.4793 |
| High ≥46 | 37.5 | – | 68.18 | 75 | 20.45 | 18.18 | ||||||
| SOFA | ||||||||||||
| Low <8 | 19.35 | 0.2474 | 67 | 0.1799 | 28 | <0.0001 | 48.39 | 0.0017 | 6.45 | 0.1285 | 9.68 | 0.4526 |
| High ≥8 | 33.33 | – | 88.89 | 88.89 | 22.22 | 18.52 | ||||||
| PSI | ||||||||||||
| Low <107 | 14.71 | 0.0291 | 67 | 0.0101 | 50 | 0.2278 | 63.46 | 0.7596 | 9.43 | 0.0827 | 15.69 | 0.6704 |
| High ≥107 | 39.39 | 15 | 71.43 | 57.14 | 28.57 | 7.14 | ||||||
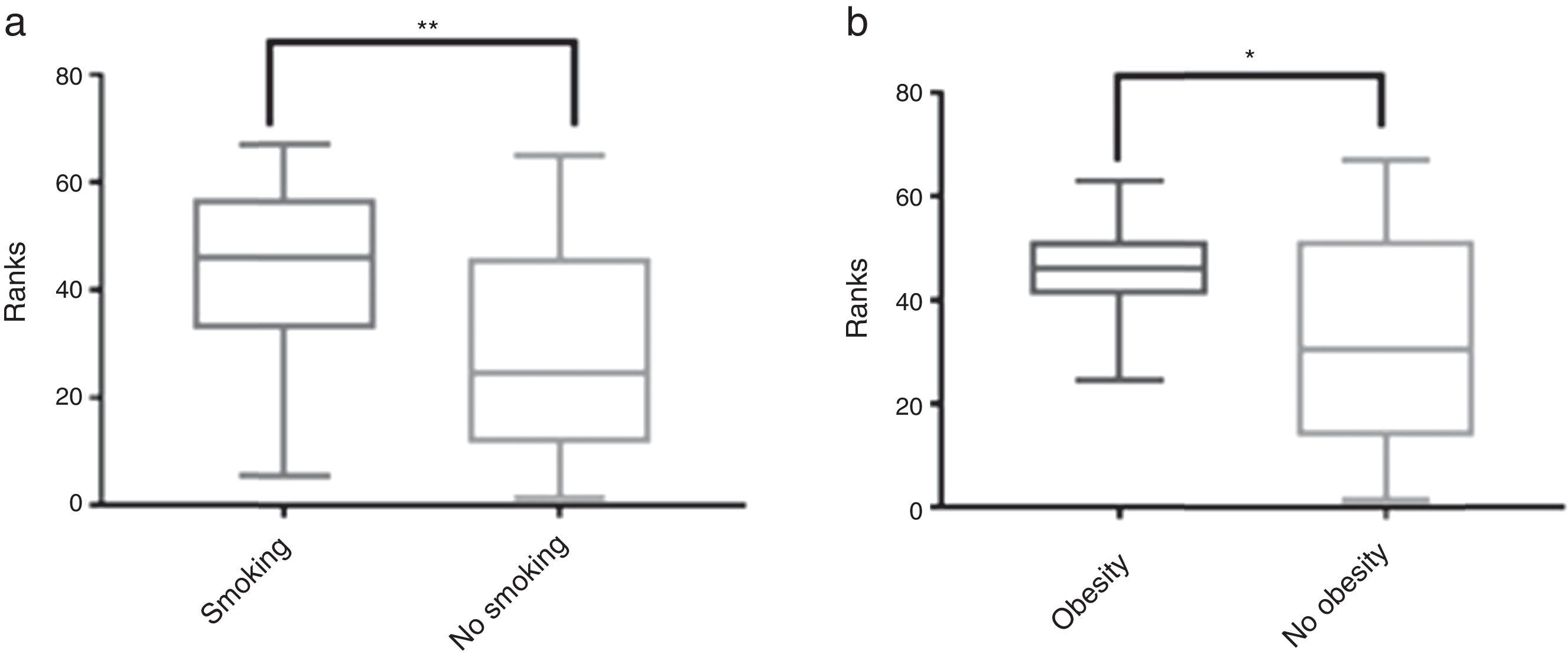
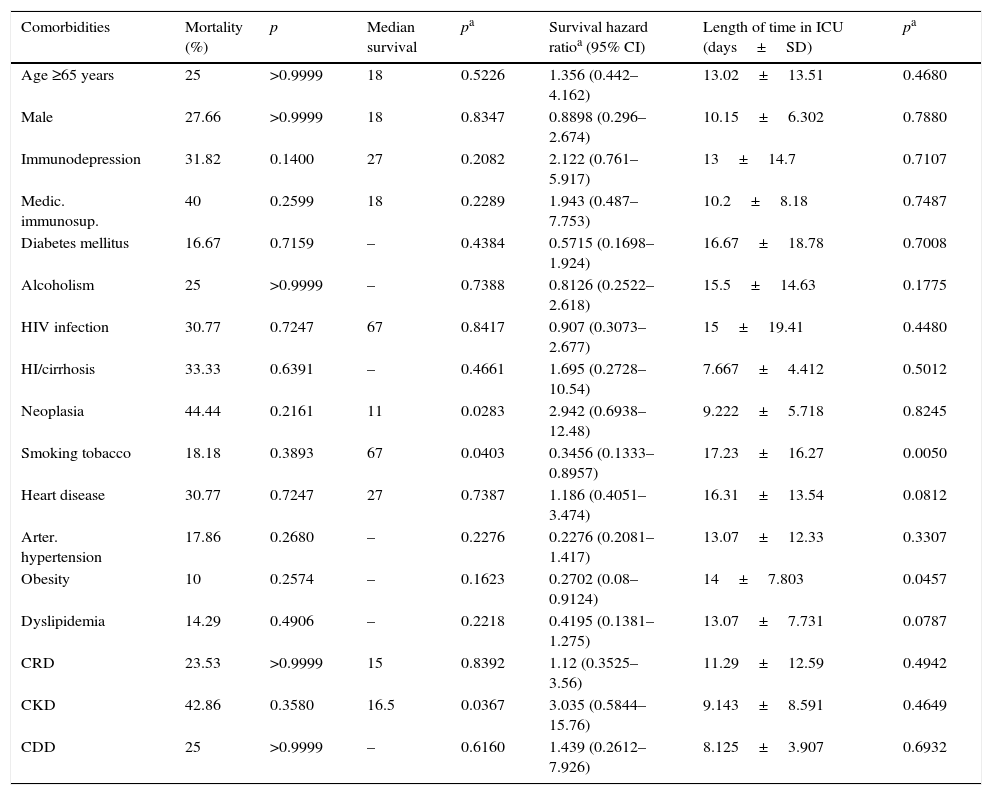
The influence of comorbidities on mortality, median survival time during patients’ time in hospital and ICU admission time is described in Table 3. Individually, comorbid conditions showed no direct impact on mortality. ICU admission time was significantly increased in obese people (14 days vs 11.84 days, p=0.0457) and smokers (17.23 days vs 9.689 days, p=0.005) (Fig. 1). Median survival time was increased in smokers (67 vs 27, p=0.0403).
Impact of demographic data and comorbidities on mortality, median survival and length of time in hospital (n=67).
| Comorbidities | Mortality (%) | p | Median survival | pa | Survival hazard ratioa (95% CI) | Length of time in ICU (days±SD) | pa |
|---|---|---|---|---|---|---|---|
| Age ≥65 years | 25 | >0.9999 | 18 | 0.5226 | 1.356 (0.442–4.162) | 13.02±13.51 | 0.4680 |
| Male | 27.66 | >0.9999 | 18 | 0.8347 | 0.8898 (0.296–2.674) | 10.15±6.302 | 0.7880 |
| Immunodepression | 31.82 | 0.1400 | 27 | 0.2082 | 2.122 (0.761–5.917) | 13±14.7 | 0.7107 |
| Medic. immunosup. | 40 | 0.2599 | 18 | 0.2289 | 1.943 (0.487–7.753) | 10.2±8.18 | 0.7487 |
| Diabetes mellitus | 16.67 | 0.7159 | – | 0.4384 | 0.5715 (0.1698–1.924) | 16.67±18.78 | 0.7008 |
| Alcoholism | 25 | >0.9999 | – | 0.7388 | 0.8126 (0.2522–2.618) | 15.5±14.63 | 0.1775 |
| HIV infection | 30.77 | 0.7247 | 67 | 0.8417 | 0.907 (0.3073–2.677) | 15±19.41 | 0.4480 |
| HI/cirrhosis | 33.33 | 0.6391 | – | 0.4661 | 1.695 (0.2728–10.54) | 7.667±4.412 | 0.5012 |
| Neoplasia | 44.44 | 0.2161 | 11 | 0.0283 | 2.942 (0.6938–12.48) | 9.222±5.718 | 0.8245 |
| Smoking tobacco | 18.18 | 0.3893 | 67 | 0.0403 | 0.3456 (0.1333–0.8957) | 17.23±16.27 | 0.0050 |
| Heart disease | 30.77 | 0.7247 | 27 | 0.7387 | 1.186 (0.4051–3.474) | 16.31±13.54 | 0.0812 |
| Arter. hypertension | 17.86 | 0.2680 | – | 0.2276 | 0.2276 (0.2081–1.417) | 13.07±12.33 | 0.3307 |
| Obesity | 10 | 0.2574 | – | 0.1623 | 0.2702 (0.08–0.9124) | 14±7.803 | 0.0457 |
| Dyslipidemia | 14.29 | 0.4906 | – | 0.2218 | 0.4195 (0.1381–1.275) | 13.07±7.731 | 0.0787 |
| CRD | 23.53 | >0.9999 | 15 | 0.8392 | 1.12 (0.3525–3.56) | 11.29±12.59 | 0.4942 |
| CKD | 42.86 | 0.3580 | 16.5 | 0.0367 | 3.035 (0.5844–15.76) | 9.143±8.591 | 0.4649 |
| CDD | 25 | >0.9999 | – | 0.6160 | 1.439 (0.2612–7.926) | 8.125±3.907 | 0.6932 |
Medic. immunosup, Medical immunossupression; HI/cirrhosis, hepatic insufficiency/cirrhosis; Arter. hypertension, arterial hypertension; CRD, chronic respiratory disease; CKD, chronic kidney disease; CDD, chronic digestive disease.
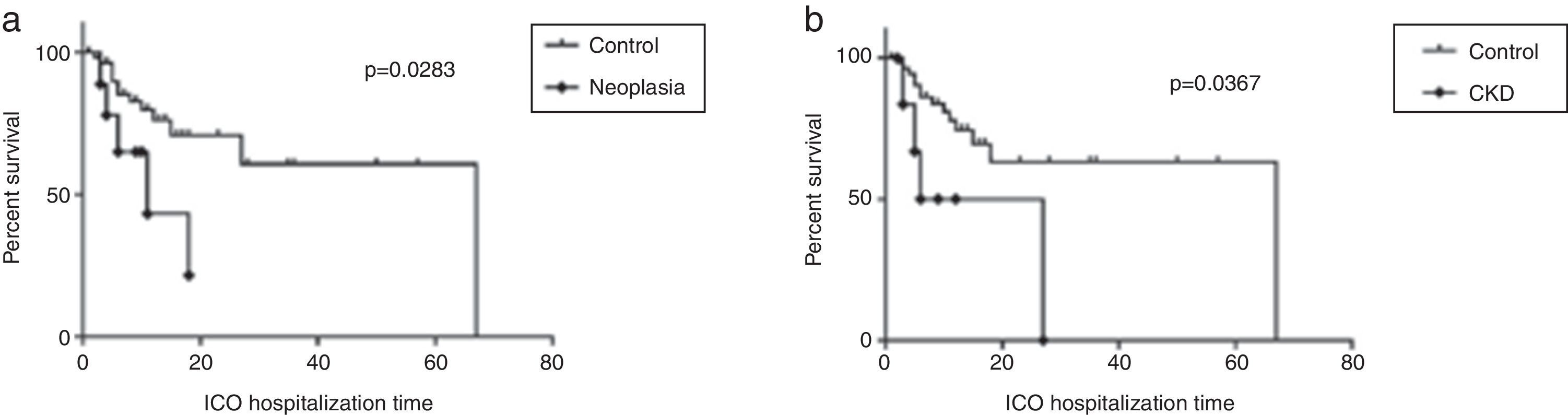
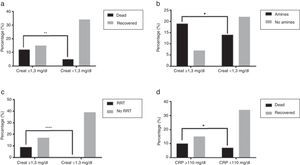
Patients with CKD and active malignant neoplasia had significantly lower median survival times than other patients (16.5 days vs 67 days, p=0.0367 and 11 days vs 67 days, p=0.0283, respectively) (Fig. 2a and b).
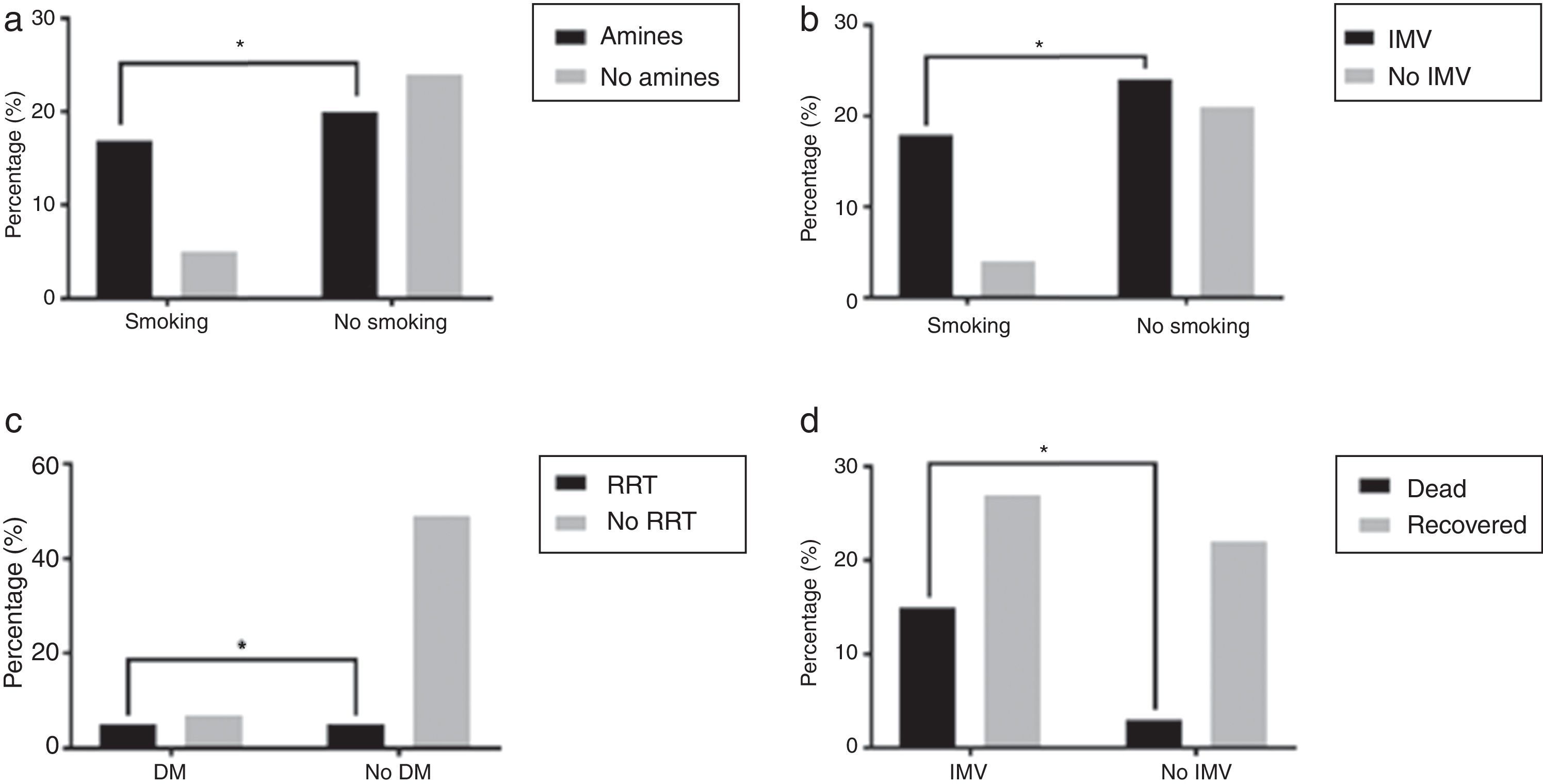
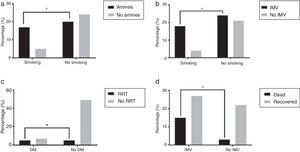
Organ support and outcomeOverall, in 85.1% of the patients some respiratory support was needed, with 62.7% patients requiring intubation and mechanical ventilation and 48.5% of the patients treated, at least initially, with NIV. 43.75% of those that initially did NIV needed intubation and mechanical ventilation. As for other dysfunctions, 56.1% patients needed vasopressor support, 15.2% patients needed renal replacement support and 14.9% had refractory hypoxemia and were put on ECMO. Some comorbid conditions were related to the need for organ support as in the case of the association of smokers and vasopressor support (77.27%, p=0.0185) and IMV (81.82%, p=0.0318) when compared to non-smokers (Fig. 3a and b). Patients with DM needed more RRT (41.67% vs 9.26%, p=0.0134) (Fig. 3c) even though only 3.42% of these patients had reported CKD diagnosis previous to being admitted. Patients who required IMV had higher mortality rate than those who did not need ventilatory support or just needed NIV (35.71% vs 12%, p=0.0466) (Fig. 3d). Patients who started ventilator support by NIV had 15.63% mortality rate.
(a) Greater need of vasopressor support in smokers, *p=0.0185; (b) greater need of invasive mechanic ventilation in smokers, *p=0.0318; (c) greater need of renal replacement treatment in diabetic patients, *p=0.0134 and (d) greater mortality in patients needing invasive mechanic ventilation, p=0.0466.
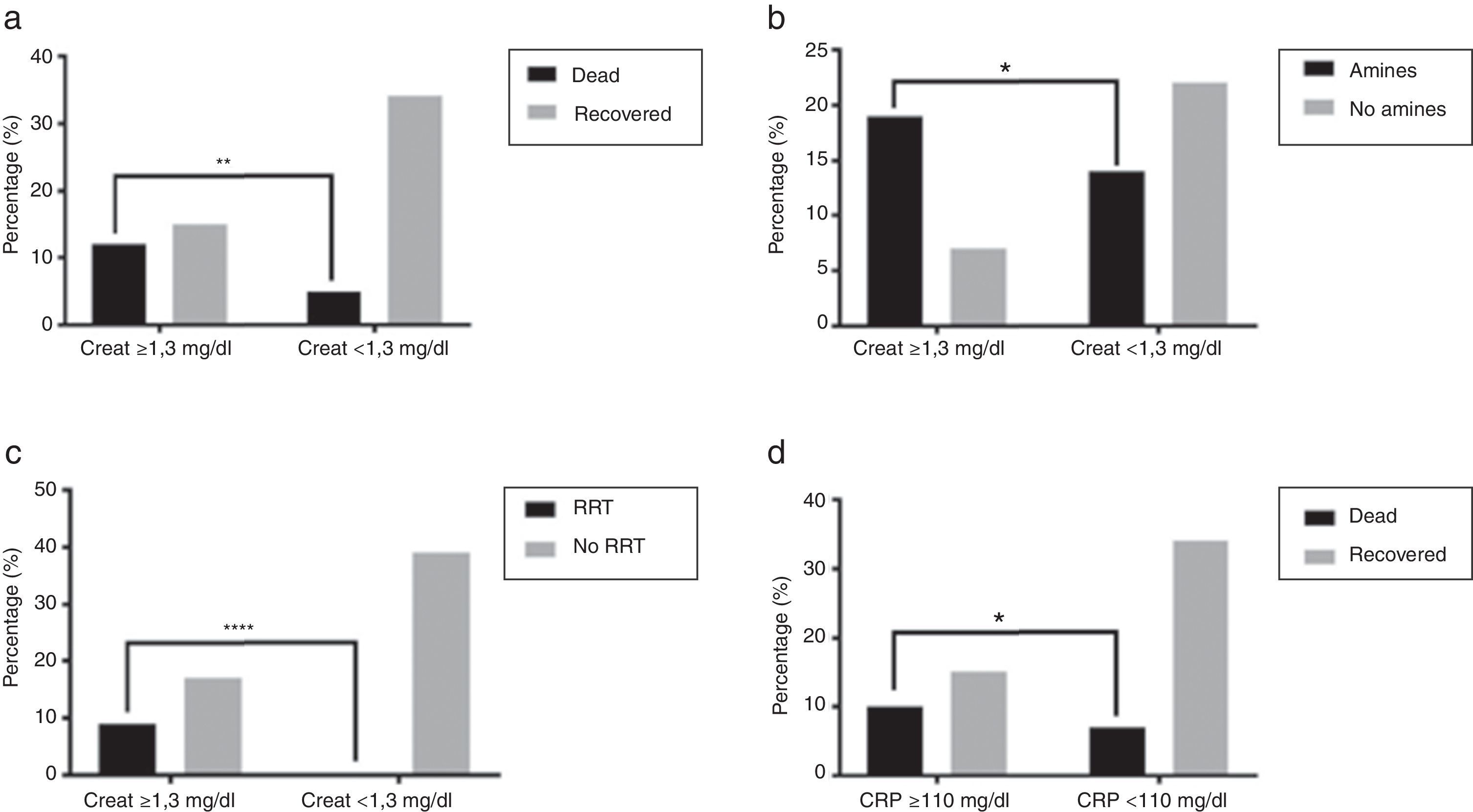
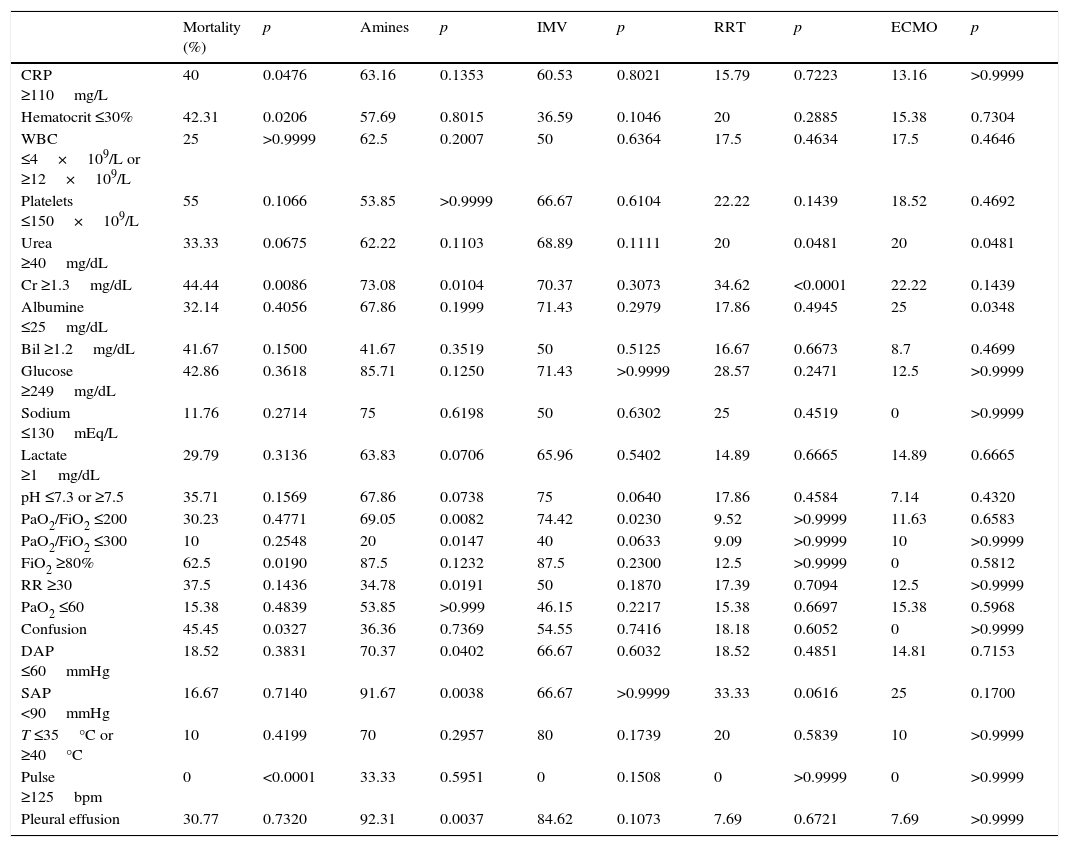
The relationship of clinical and analytic parameters and mortality and treatment during hospital admission is described in Table 4. Higher mortality rates were found in patients with CRP ≥110mg/L (40% vs 17.7%, p=0.0476), Ht ≤30% (42.31% vs 15%, p=0.0206), Cr ≥1.3mg/dL (44.44% vs 12.82%, p=0.0086) (Fig. 4a), FiO2 ≥80% (62.5% vs 18.6%, p=0.0190) and who showed confusion (45.45% vs 13.16%, p=0.0327) when admitted at ICU. The need of vasopressor support was associated with Cr ≥1.3mg/dL (73.08% vs 38.89%, p=0.0104) (Fig. 4b), PaO2/FiO2 ratio ≤200 (69.05% vs 23.08%, p=0.0082), and presence of pleural effusion (92.31% vs 45.1%, p=0.0037). Patients with PaO2/FiO2 ≤200 were associated to a greater need of IMV (74.42% vs 38.46, p=0.0230).
Relationship of clinical and analytic parameters at admission with mortality and treatment support.
| Mortality (%) | p | Amines | p | IMV | p | RRT | p | ECMO | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| CRP ≥110mg/L | 40 | 0.0476 | 63.16 | 0.1353 | 60.53 | 0.8021 | 15.79 | 0.7223 | 13.16 | >0.9999 |
| Hematocrit ≤30% | 42.31 | 0.0206 | 57.69 | 0.8015 | 36.59 | 0.1046 | 20 | 0.2885 | 15.38 | 0.7304 |
| WBC ≤4×109/L or ≥12×109/L | 25 | >0.9999 | 62.5 | 0.2007 | 50 | 0.6364 | 17.5 | 0.4634 | 17.5 | 0.4646 |
| Platelets ≤150×109/L | 55 | 0.1066 | 53.85 | >0.9999 | 66.67 | 0.6104 | 22.22 | 0.1439 | 18.52 | 0.4692 |
| Urea ≥40mg/dL | 33.33 | 0.0675 | 62.22 | 0.1103 | 68.89 | 0.1111 | 20 | 0.0481 | 20 | 0.0481 |
| Cr ≥1.3mg/dL | 44.44 | 0.0086 | 73.08 | 0.0104 | 70.37 | 0.3073 | 34.62 | <0.0001 | 22.22 | 0.1439 |
| Albumine ≤25mg/dL | 32.14 | 0.4056 | 67.86 | 0.1999 | 71.43 | 0.2979 | 17.86 | 0.4945 | 25 | 0.0348 |
| Bil ≥1.2mg/dL | 41.67 | 0.1500 | 41.67 | 0.3519 | 50 | 0.5125 | 16.67 | 0.6673 | 8.7 | 0.4699 |
| Glucose ≥249mg/dL | 42.86 | 0.3618 | 85.71 | 0.1250 | 71.43 | >0.9999 | 28.57 | 0.2471 | 12.5 | >0.9999 |
| Sodium ≤130mEq/L | 11.76 | 0.2714 | 75 | 0.6198 | 50 | 0.6302 | 25 | 0.4519 | 0 | >0.9999 |
| Lactate ≥1mg/dL | 29.79 | 0.3136 | 63.83 | 0.0706 | 65.96 | 0.5402 | 14.89 | 0.6665 | 14.89 | 0.6665 |
| pH ≤7.3 or ≥7.5 | 35.71 | 0.1569 | 67.86 | 0.0738 | 75 | 0.0640 | 17.86 | 0.4584 | 7.14 | 0.4320 |
| PaO2/FiO2 ≤200 | 30.23 | 0.4771 | 69.05 | 0.0082 | 74.42 | 0.0230 | 9.52 | >0.9999 | 11.63 | 0.6583 |
| PaO2/FiO2 ≤300 | 10 | 0.2548 | 20 | 0.0147 | 40 | 0.0633 | 9.09 | >0.9999 | 10 | >0.9999 |
| FiO2 ≥80% | 62.5 | 0.0190 | 87.5 | 0.1232 | 87.5 | 0.2300 | 12.5 | >0.9999 | 0 | 0.5812 |
| RR ≥30 | 37.5 | 0.1436 | 34.78 | 0.0191 | 50 | 0.1870 | 17.39 | 0.7094 | 12.5 | >0.9999 |
| PaO2 ≤60 | 15.38 | 0.4839 | 53.85 | >0.999 | 46.15 | 0.2217 | 15.38 | 0.6697 | 15.38 | 0.5968 |
| Confusion | 45.45 | 0.0327 | 36.36 | 0.7369 | 54.55 | 0.7416 | 18.18 | 0.6052 | 0 | >0.9999 |
| DAP ≤60mmHg | 18.52 | 0.3831 | 70.37 | 0.0402 | 66.67 | 0.6032 | 18.52 | 0.4851 | 14.81 | 0.7153 |
| SAP <90mmHg | 16.67 | 0.7140 | 91.67 | 0.0038 | 66.67 | >0.9999 | 33.33 | 0.0616 | 25 | 0.1700 |
| T ≤35°C or ≥40°C | 10 | 0.4199 | 70 | 0.2957 | 80 | 0.1739 | 20 | 0.5839 | 10 | >0.9999 |
| Pulse ≥125bpm | 0 | <0.0001 | 33.33 | 0.5951 | 0 | 0.1508 | 0 | >0.9999 | 0 | >0.9999 |
| Pleural effusion | 30.77 | 0.7320 | 92.31 | 0.0037 | 84.62 | 0.1073 | 7.69 | 0.6721 | 7.69 | >0.9999 |
CRP, C-reactive protein; WBC, white blood cells; leucocytes; Cr, creatinine; Bil, bilirrubine; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; RR, respiratory rate; DAP, diastolic arterial pressure; SAP, systolic arterial pressure; T, temperature.
The patients admitted to ICU with severe CAP were mostly men (70.15%) admitted with respiratory failure (82.09%) and septic shock (10.45%) with a global mortality rate of 26.87%, which confirms data from other studies.1,9,12 For patients with ARDS, mortality rate was 29.09% reinforcing ARDS association with appreciable mortality with estimates ranging from 26 to 58%.5 In patients with septic shock at admission, mortality rate was higher, 42.86%. In fact, sepsis is still considered the first leading cause of death among patients in non-coronary medical ICUs, with respiratory tract infections being the most common cause.19
The mean age of patients was 55.97% and only 29.85% were older than 65. Although it was expected a greater frequency and severity of the disease in the elderly, mortality rate was similar in patients under or over 65 years old (p>0.9999).2,8,22,31 So, age per se did not have an impact on prognosis10–12,31 and this could be explained by the increased number of patients younger than 65 with comorbidities. In fact, the mean age of patients with HIV infection and/or chronic digestive disease and/or smoking habits was lower than 52. The majority of the patients (94.03%) had one or more comorbid conditions reinforcing the impact of chronic subjacent conditions as a risk factor in CAP.23,24
Hospital admissions during winter over a period of 3 months represented 50.75% of the cases, which is in conformity with a seasonal variation of CAP.1
In 47.27% of the patients an etiologic agent was not isolated, as seen in other studies.3,12S. pneumoniae was the most frequently identified agent (13.43%), which emphasizes the potential severity of this pathogen and the requirement for ICU admission.1 H1N1 was present in 8.96% patients and in one of these patients S. pneumoniae co-infection occurred, reinforcing the importance of the evaluation for secondary bacterial infection in severely ill patients with influenza pneumonia.1P. jirovecci was found in 7.46% patients, all HIV positive, although its incidence seems to be decreasing due to antiretroviral therapy.1 Since S. pneumoniae continues to be one of the more often isolated agents, it might be important to insist on vaccine promotion following CDC recommendations to vaccinate children under 2 years old, people from the age of 65, patients with comorbidities of any age and smokers.6 Portuguese DGS (Direção Geral de Saúde) guidelines recommend universal antipneumococcal immunization since January 2017 for all children under 5 years old and people aged 65 and older and also considers patients at risk for invasive pneumococcal disease that should be vaccinated regardless of their age.29
Several severity indexes, two CAP scores and three ICU scores were used to estimate in-hospital mortality. Patients classified as high grade APACHE II and PSI scores had significantly higher mortality rates and smaller median survival times. Other scores did not show significant differences between high grade and low grade patients even though they followed the same tendency. The lack of ability to predict mortality by SAPS II and SOFA scores in contemporary ICU cohorts may reflect their need for re-calibration. Missing values for some variables, the use of the worst recorded parameters over a 24h period regardless of treatment and inclusion of the Glasgow Coma Scale when assessing sedated and mechanically ventilated patients may justify the inaccuracy of these models. Also, the SOFA score was not designed for mortality prediction but to evaluate organ dysfunction and morbidity.33 On the other hand, the fact that just the APACHE II and the PSI system scores showed statistical differences highlights the hypothesis that mortality prediction may be related to prior health conditions. Apart from these two score systems, only the SAPS II takes prior health conditions into consideration and, although its results were not statistically significant, it was the third more accurate system predicting mortality. Global mortality rate in this sample was 26.87%, approximately 1.5 times higher than the mortality risk estimated by APACHE II (41.38%) and PSI (39.39%) scores for high score patients. In reality, severity index systems are not 100% accurate. They generally consider a variety of parameters that vary among different score systems and pretend to predict the likelihood of in-hospital mortality and to be a guide to decide the initial phase of treatment.25,31 However, they do not take into account important parameters such as social factors or performance status. Also, the selection of the median score to group patients into low grade or high grade scores can have influenced these results. Besides helping to predict risk of in-hospital mortality, the ICU scores could also predict severity and the need for organ support such as cardiovascular and respiratory support. SMART-COP is another simple, practical clinical score that allows the identification of patients with severe pneumonia who require ICU care for eventual invasive ventilation and vasopressor support.30 In this study, several clinical and analytical parameters were related to greater mortality and the need for organ support, but most of them were already considered by previous scores except CRP and albumin levels. The importance of identifying sepsis early and to initiate prompt measures to minimize complications is unanimous. The SIRS criteria is too sensitive but not sufficiently specific for this purpose. The Third International Consensus Definitions for Sepsis recommended qSOFA as a quicker and more specific way to identify infected patients outside the ICU who are likely to be septic and at a greater risk of death or prolonged intensive care unit stay.20 Although qSOFA is less accurate than the original SOFA score, it does not require laboratory tests and it is simpler to assess.21
Comorbidities showed no individual impact on mortality. They were evaluated by a qualitative analysis, valuing its presence or absence. It would be relevant to access comorbid conditions in a future study by quantifying the grade of disease and perform a multivariate analysis to better understand comorbidities’ cumulative behavior. Another limitation of this study was the inability to determine symptoms’ duration until treatment. Current more aggressive treatment options and precocity of adequate treatment may contribute to lesser mortality even in the presence of comorbidities.25,31 On the other hand, a prolonged ICU admission time in obese patients and smokers was found, as previously reported for obesity, besides an apparent paradoxical link between obesity and improved survival.28 According to previous studies, the length of patients’ stay in hospital seems to be influenced by several variables such as the PSI score, associated comorbidities and the presence of clinical complications.7 Smoking is an extensive studied comorbidity, known for the worst prognosis, including higher mortality rates.16 In this cohort, smokers needed more vasopressor support and IMV than other patients and, although requiring a longer stay in hospital, they had a higher median survival time. It would make sense to do a study on the connection between the number of cigarettes smoked per day and the length of the smoking habit. These two comorbidities are potentially modifiable conditions leading to the importance of patient information and promotion of healthy habits. DM was associated with a greater need for RRT, probably due to the worsening of pre-existing clinical or subclinical kidney and/or cardiovascular diseases.27 Although higher mortality seen in patients with CKD did not reach statistical significance (p=0.2161), patients with chronic kidney disease showed lower median survival time during their time at hospital and, similarly to what happens with diabetic patients, this may reflect a greater susceptibility to develop an acute kidney failure.25,27 Acute renal impairment had an important interference in patient evolution in this study as already proposed by previous studies.25 Active malignant neoplasia was also associated with lower median survival time. Although considered a risk factor for CAP,1 immunosuppression in general had no impact on prognosis. Once again, it would be relevant to develop a multivariate study to understand how co-morbid conditions influence treatment decisions.
IMV was related to a significantly higher mortality rate, confirming other results that link ARDS and IMV with a higher mortality rate.5,9 Although more than half a patients that initially undergo NIV were successfully treated, 43.75% needed further intubation and mechanical ventilation. This fact enhances NIV's controversial role in severe acute respiratory failure due to CAP, although there have been technological improvements of ventilatory equipments.32
Several clinical and analytic parameters were significantly related to higher mortality rates (Table 4). The majority of the studied parameters are considered by the well-established severity index systems that estimate mortality risk, except for C-reactive protein and albumin. As such, it would be relevant to promote further studies to investigate the predictive value of these two specific parameters.
ConclusionsEtiologic diagnosis was not done in 46% of the patients and, as expected, S. pneumoniae was the most common agent of severe CAP, reinforcing the importance of pneumococcal vaccine.
Smoking tobacco and obesity, both potentially modifiable conditions, were associated with prolonged hospitalization stay, although not associated with increased mortality.
In general, patients in need of invasive ventilation support and those who present acute renal failure had a worse prognosis.
It would be advisable to develop a prospective study with a higher number of patients, allowing a multivariable analysis with quantitative characterization of each comorbid condition and its impact on mortality.
Scores are a helpful decision guide but they all have their specific limitations.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Alcina Ferreira for the help in collecting patient data and Aura Silva for the support on statistical analysis.