Chronic anal fissure is a common condition associated with intense pain. Local botulinum toxin injection is a valid option in its management. The purpose of this study was to evaluate the efficacy of botulinum toxin on pain relief in chronic anal fissure patients.
MethodsWe conducted a retrospective cohort study, involving 81 consecutive patients referred to a chronic pain management unit due to a chronic anal fissure for treatment with botulinum toxin, during a 4 year period. Data were collected from hospital records regarding pre-treatment and post-treatment pain (numeric rating scale), side effects, need for botulinum toxin reinjection and need for surgical treatment. We used standard statistical methods for inter (t-test and qui2) and intra-group (paired sample t-test) comparisons, according to variables distribution.
ResultsPain intensity rest score significantly improved after BoNT injection [variation: −4.2±2.9 (p<0.001)], as did pain post-defecation score [variation: −5.1±3.0 (p<0.001)]. 8.6% needed botulinum toxin reinjection and 23.5% were submitted to surgery. Side effects were reported in 8.6%.
DiscussionThe efficacy of botulinum toxin use on pain reduction along with its non-permanent and minor side effects support its role in the resolution of chronic anal fissure. However, treatment failure in the long term is still significant.
ConclusionBotulinum toxin is effective on pain relief in patients with chronic anal fissure, which supports its inclusion in the management algorithm of this condition.
Anal fissure is a tear localized at the distal part of the anal canal.1 Chronicity of this condition is often defined as a history of pain lasting more than 6 weeks.1
It usually occurs between the ages of 20 and 40 with an equal distribution between men and women and an estimated lifetime incidence of about 8–11%.1,2 Most of the fissures are localized in the posterior midline.1,2
Its pathophysiology includes several pathogenic factors. Constipation, resulting in hard and bulky stools usually triggers the initial event of a tear in the anoderm.3 The persistence of the fissure is justified by a vicious circle involving the hypertonia/spasm of the internal anal sphincter and pain caused by the ulceration and the passage of stools. Local ischemia of the anoderm also contributes to the non-healing of the fissure.3
Thus, most treatments, currently proposed, are aimed at achieving the relaxation of the internal anal sphincter.4 Conservative treatment is usually recommended as an initial approach and surgical treatment is usually reserved for refractory cases.4,5
Numerous conservative therapeutic alternatives have been proposed, such as topical nitrates. Their role as inhibitory neurotransmitters in the relaxation of the internal sphincter is still being investigated and their prolonged use provides fissure healing in some patients.4,6 However, adverse effects (anal burning, headaches and hypotension) and escape phenomena (tachyphylaxis) often limits their prescription.4,6
Surgery of the internal anal sphincter (irreversible lateral sphincterotomy) results in pain relief and fissure healing in more than 95% of cases.4,5 However, it can also induce fecal incontinence in up to 14% of patients and its costs are significantly higher than its conservative counterparts.7,8
In that line of thought, injecting botulinum toxin (BoNT) into the anal internal sphincter eliminates the muscle spasm by blockage of neurotransmission, inducing a reversible chemical sphincterotomy while breaking the pain-spasm-pain cycle.9–12 BoNT injection for the treatment of chronic anal fissure has also been proven effective in alleviating anal pain.13 It constitutes a viable alternative and several studies have been conducted to investigate its real value in the management of this specific condition.9–12
It has also been used in combination with other conservative treatments14 or with less aggressive surgical treatments in order to try to maximize its effect and diminish the risks associated with classical surgeries.15
The main purpose of this study was to evaluate the efficacy of BoNT on pain relief in patients with chronic anal fissure. Secondarily the measures of treatment failure and morbidity were also investigated.
MethodsWe conducted a retrospective cohort study, involving 81 consecutive patients referred to a chronic pain management unit, by their assisting surgeon, due to a chronic anal fissure, for treatment with BoNT injection. Patients were recruited between January 2009 and December 2013.
Demographic and clinical data were collected from hospital records. Pre-treatment (T1) and 4 weeks post-treatment (T2) pain was accessed using a numeric rating scale (NRS) score (rest and post-defecation). Information on side effects, need for BoNT reinjection and/or surgical lateral sphincterotomy, was also retrospectively obtained.
The treatment consisted of 40U of lyophilized BoNT (OnabotulinumtoxinA) diluted in 1ml of isotonic saline solution. The internal anal sphincter was manually identified and injection was done using a 25-G needle, by three spots, two, lateral to the fissure (10U+10U) and another 20U anteriorly. All patients were reevaluated within 4 weeks of BoNT treatment. Patients were then followed by a general surgeon who verified the resolution of the symptoms or the need for additional treatment.
We defined non-responders as those needing subsequent treatment (BoNT reinjection and/or surgical lateral sphincterotomy) during the 12 months that followed the initial BoNT injection.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS® version 21). We used mean and standard deviation to describe continuous variables and proportions for categorical variables. For pain score variation evaluation, we used the paired sample t-test for intra-group comparison. For comparison between the responder and non-responder groups we utilized t-test for continuous variables and qui2 testing for categorical variables.
ResultsOf the 81 patients studied, the majority were male 44 (54.3%) with a mean age of 49.6±15.3 years.
Only 66 (81.5%) had complete data for NRS (before and after BoNT). Those excluded were younger (mean age: 43.7±15.7 years) and more frequently female (66.7%). NRS rest score significantly improved after BoNT injection [T1: 5.2±2.9; variation: −4.2±2.9 (p<0.001)], as did NRS post-defecation score [T1: 7.4±2.5; variation: −5.1±3.0 (p<0.001)] (Table 1).
Pain scores variation after BoNT treatment.
| NRS* score | Rest NRS T1 | Rest NRS T2 | Rest NRS T1–T2 | p value | Def. NRS T1 | Def. NRS T2 | Def. NRS T1–T2 | p value |
|---|---|---|---|---|---|---|---|---|
| Mean | 5.2 | 1.0 | 4.2 | <0.001** | 7.4 | 2.3 | 5.1 | <0.001** |
| Std. Dev. | 2.9 | 1.8 | 2.9 | 2.5 | 2.4 | 3.0 |
Bold values represent pain scores variation.
NRS: numeric rating scale; Std. Dev.: standard deviation; Def.: defecation; T1: before treatment; T2: 4 weeks after treatment.
Most patients had previously undergone other conservative treatment strategies including bulk aperients (bran or other forms of fiber), sitz baths and topical agents such as nitrates, calcium channel inhibitors or anesthetics. None of these treatments were successful in healing the fissure before the use of BoNT. There was no concomitant treatment with other medications during or after treatment with botulinum toxin.
A total of 23 (28.3%) non-responders were identified, 7 (8.6%) needed a BoNT reinjection of which 4 needed subsequent surgical treatment. In the end 19 (23.5%) patients were submitted to surgery.
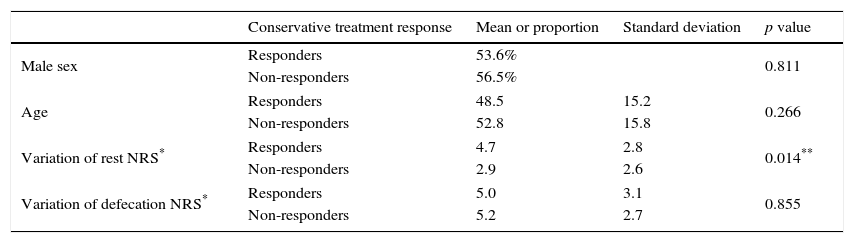
There were no differences between groups (responders vs non-responders) regarding demographic characteristics and post-defecation NRS variation. However, there was significant difference in the improvement of rest NRS score favoring the responder group (Table 2).
Comparison between responder and non-responder groups.
| Conservative treatment response | Mean or proportion | Standard deviation | p value | |
|---|---|---|---|---|
| Male sex | Responders | 53.6% | 0.811 | |
| Non-responders | 56.5% | |||
| Age | Responders | 48.5 | 15.2 | 0.266 |
| Non-responders | 52.8 | 15.8 | ||
| Variation of rest NRS* | Responders | 4.7 | 2.8 | 0.014** |
| Non-responders | 2.9 | 2.6 | ||
| Variation of defecation NRS* | Responders | 5.0 | 3.1 | 0.855 |
| Non-responders | 5.2 | 2.7 | ||
NRS: numeric rating scale.
Side effects of BoNT treatment were reported in 7 (8.6%) patients, and consisted of transient flatus incontinence (2), minor fecal soiling (3) and minor hemorrhage (2).
DiscussionThe role of hypertonicity and spasm of the internal anal sphincter in the persistence of anal fissures has been well established and thus focus has been on methods of reducing sphincter overactivity.4
Lateral internal sphincterotomy is still regarded as the gold standard treatment for chronic fissures, despite known potential for serious morbidity, namely fecal incontinence.5,7 Therefore, a search for less invasive procedures has been ongoing, including topical nitrates and botulinum toxin injections. However, nitrates are poorly tolerated due to their association with headaches.4,6
This chemical denervation is not permanent and the clinical efficacy generally lasts for 2–3 months, which is enough time for sphincter resting pressure reduction to allow for healing.4,9
As previously stated, a lot has been written about the role of hypertonicity and spasm of internal anal sphincter but less so about the specific role of pain in this condition. Pain is a commonly used surrogate for treatment response in these patients.13 To allow for better characterization of severity and persistence of pain we used both rest and post-defection pain scores. As we can see, pain is more intense in the post-defecation period. Use of BoNT successfully reduced pain scores in both situations at the 4 week point.
BoNT has been used off-label to treat other painful conditions although its use is widely considered controversial. It has been used off-label for treating migraines, cervical dystonia, thoracic outlet syndrome, and piriformis syndrome.16 New data suggest a mechanism of action for this analgesic effect of BoNT treatment in that the delivered BoNT exerts not only local action on sensory afferent terminals (blocking the release of various pain-modulating neurotransmitters, including glutamate, substance P and calcitonin gene-related peptide) but might also modulate central afferent cell bodies and spinal dorsal horn terminals, where they cleave SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins and block transmitter release.17,18
The fact that the BoNT might have an analgesic influence in addition to its relaxing consequence on the hypertonic muscle could help explain its overall positive effect in breaking pain-spasm-pain vicious cycle that characterizes the chronicity of this condition.
Nonetheless, there was a significant rate of non-responders (29.1%), which is in accordance with what is currently known for this kind of treatment.4,9–12
The non-responder and responder groups presented comparable pain scores improvement at four weeks after BoNT in post-defecation pain score. However rest pain score improved more significantly in the responder group, a fact that might support the use of this variable as a measure of long-term treatment efficacy.
On the other hand, although pain is often considered a surrogate for anal fissure activity, macroscopic healing is also an important determinant of resolution or need for further treatment and these data are not available in our study.
In addition, a recent study has postulated the presence of a rapid low amplitude contraction “anal sphincter fibrillation” in a subset of these patients that might be predictive of BoNT treatment success, although further confirmation is still warranted.19
Reported side-effects were similar to other studies and usually mild, uncommon and temporary.4,9
This study adds to current evidence that BoNT is a valid and safe therapeutic option for chronic anal fissure and that it is effective in significantly reducing anal pain. Sample size and heterogeneity of presentation support external validity of the results.
However, this was a retrospective analysis of a single cohort of BoNT treated chronic anal fissures, pain was the only direct outcome measure used to assess treatment response and it was only assessed at four weeks post-treatment.
In conclusion BoNT appears to be effective on pain relief in patients with chronic anal fissure, which may further support its use as a treatment included in the management algorithm of this condition. However, in spite of uncommon and transient side effects, treatment failure in the long term is still significant.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Dr. Afonso Rocha, for his prominent role in the review of this paper and also for his contribute in the process of statistical analysis.
We would also like to acknowledge Dr. Fernando Parada for his continuous and unwavering support of the scientific production in the Physical and Rehabilitation Department.