As clinical oncology evolves with new treatment options becoming available, there is an increasing demand on anatomic imaging for the assessment of patients at different stages. Imaging with hyperpolarized 13C-labelled cell substrates has the potential to become a powerful tool in many steps of clinical evaluation, offering a new metabolic metric and therefore a more personalised approach to treatment response. This articles explores the metabolic basis and potential for translation of hyperpolarised pyruvate as a dynamic nuclear polarisation probe in clinical oncology.
New advances in a wide range of fields from genomics to medical imaging are allowing patients to be treated and monitored more precisely and effectively and in ways that better meet their individual needs. Development of tools to truly personalise healthcare will improve our ability to predict and account for individual differences in disease diagnosis, experience, and therapy response. An increased understanding of the relationship between molecular knowledge and conventional anatomical imaging, using molecular imaging (MI), is allowing diagnostic imaging to become more specific and sensitive. The ability to non-invasively assess metabolism in vivo and in real time was made possible recently in humans, for the first time, through the use of dynamic nuclear polarisation (DNP) of 13C-labelled metabolites, which could be detected in vivo using magnetic resonance spectroscopic imaging (MRSI). This article explores the metabolic basis and potential for translation of [1-13C]pyruvate as a DNP probe in clinical oncology. A subset of cancers is also discussed.
Metabolic changes in cancer: probing glycolysis with hyperpolarized [1-13C]pyruvateMetabolic reprogramming, namely increased glycolysis, is a well-recognised and highly prevalent hallmark of cancer.1,2 Since its first report in cancer cells under aerobic conditions, by Otto Warburg in the 1930s,3,4 numerous additional experimental observations have verified this same phenomenon, which was named the “Warburg effect” over the following decades.5 From a bioenergetic standpoint glycolysis is an inefficient process to produce energy as it makes only 2 adenosine triphosphate (ATP) molecules per glucose molecule compared to the 38 ATP molecules that complete oxidation produces. Additionally, formation and release of metabolic products, such as hydrogen ions (H+), to the extracellular space leads to an acidic and potentially toxic environment for cells.6,7 At a first glance, this selection for inefficient substrate metabolism seems counterintuitive. However, a growing clinical and pre-clinical body of knowledge has revealed the relevance and advantage of this metabolic phenotype in cancer cell survival, namely in providing tumours with a powerful proliferative and invasive advantage over surrounding normal tissues.2,8 Increased glycolysis enables not only the generation of intermediates and cofactors essential for proliferation but also the alteration of the extra-cellular pH, specifically environmental acidosis, that facilitates invasion through destruction of adjacent normal cell populations, degradation of the extracellular matrix and promotion of angiogenesis.2,8
As an almost universal cancer signature, glycolysis is a very attractive pathway to probe either pre-clinically or clinically. While positron emission tomography (PET) with computed tomography (CT) has been the metabolic imaging method most familiar to clinicians, other imaging techniques based on MR also exist. [1-13C]pyruvate is the best-established metabolite in the DNP field and the only one so far that has successfully translated to the clinic.9 Another DNP probe, which also probes glycolysis, is 13C labelled glucose which allows not only assessment of glycolytic flux but also flux into the pentose phosphate pathway (PPP).10
Pyruvate has a central role in cell metabolism. In cancer, pyruvate is mostly reversibly reduced by nicotinamide adenine dinucleotide11 into lactate, in a reaction catalysed by the enzyme lactate dehydrogenase12 or transaminated by glutamate to produce alanine in the reaction catalysed by alanine transaminase (ALT).13 These reactions can be probed in vivo and in real time using hyperpolarized [1-13C]-pyruvate.
Basic principles of dynamic nuclear polarisationDNP significantly (>10,000-fold) increases the signal from and therefore the sensitivity of detection of injected 13C-labelled compounds in vivo.14 The process involves mixing 13C-labelled molecules with small quantities of a stable radical, cooling the mixture to approximately 1K in a magnetic field and irradiating the electron spins with microwave irradiation resulting in transfer of the polarisation from the fully polarised electron spins on the radical to the 13C nuclei. The compound is then rapidly dissolved in hot pressurised buffer, and can then be injected into a cell suspension or an animal or a human being in a separate imaging magnet.15,16 The hyperpolarized signal, however, is transient and lasts in vivo for about 2–3min, depending on the substrate.17
The application of this powerful approach has allowed in vivo imaging of metabolites and their enzymatic conversion into other species, at high temporal and relatively high spatial resolutions.14,18
Translation to humansOne of the most challenging milestones for hyperpolarized substrates was achieved with [1-13C]pyruvate with its successful translation to humans in a study in prostate cancer. This imaging proof-of-principle study showed not only the feasibility of HP [1-13C]pyruvate as an agent for noninvasively characterising metabolic alterations in tumours but also its safety in humans.9 However, this technique is still in its infancy and many hurdles will need to be addressed if it is to realise a clinical role within the toolkit of existing competing clinical techniques.
Biologically, a better understanding of the relationship between the DNP image data, specific enzymatic reactions and malignancy would greatly benefit the hyperpolarized field. Further advances in our understanding of cancer biology have revealed increasing tumour heterogeneity, which significantly increases in complexity in the clinical setting. One interesting point would be to depict this heterogeneity with imaging. However, again, studies on the biological-imaging relationship are needed.
The clinical application of this technique in a daily basis will also need to be refined for reliable results to be acquired. Mirroring other metabolic imaging techniques that are already used routinely in the clinic, i.e.18Fluorodeoxyglucose (18F-FDG) PET, improved variability of hyperpolarized [1-13C]-pyruvate was shown when subjects were fasted.19 However this result will have to be validated in humans.
At a technical level current research has focused on improving methods for fast imaging, analyzing kinetic data, and the development of methods for increasing the hyperpolarized signal lifetime.20 Improved hyperpolarizer designs, which use automated injection systems21 and higher field-strengths22 will improve the substrate polarisation levels at the time of injection. Further, new methods to accelerate pyruvate polarisation can also be applied.23,24 Chemical derivatization to enhance tissue uptake25 and prolongation of the polarisation lifetime by deuteration10 may also allow the use and development of new agents.
Potential of DNP in current clinical practiceFrom the very first reports HP [1-13C]pyruvate has proved to be a very promising probe in the oncology field, with great potential to be applied in many of the clinical patient management steps.26 Preclinically, hyperpolarized [1-13C]pyruvate has been reported as an interesting screening tool for early detection and secondary screening of pancreatic cancer27 (Fig. 1). In another study, [1-13C]pyruvate detected metabolic changes prior to tumour formation.28 In the clinic, increased lactate labelling was also observed in histologically confirmed areas of prostate cancer that were not identifiable by conventional 1H-MRI measurements.9 The role of hyperpolarized [1-13C]pyruvate in diagnosis and differential diagnosis between normal and cancer tissues have been widely reported, with increased lactate labelling occurring in tumour tissues.29,30 In the clinic, tumour grading by biopsy can sometimes be difficult given the location and accessibility of the organ of interest, e.g. pancreas. It is for these reasons that transfer of this technique would be interesting, as it would allow not only for more accurately targeted biopsies but also for a potential reduction in biopsy procedures under some circumstances. The few studies that have explored the role of HP [1-13C]pyruvate in grading and prognosis in the prostate have produced promising results.31,32 Changes in tumour size and burden form the major part of the Response Evaluation Criteria In Solid Tumours (RECIST) criteria, which is a widely used method for assessing treatment response in clinical trials.33 Numerous studies have demonstrated early decreases in hyperpolarized 13C label exchange between injected [1-13C]pyruvate and the endogenous lactate pool in a range of cancer models following treatment with cytotoxic chemotherapy,34,35 targeted drugs,36–38 and radiotherapy39–41 before any tumour size change was observable.
13C chemical shift images acquired with a surface coil representing [1-13C]lactate, [1-13C]alanine and [1-13C]pyruvate peak intensities. Differential anatomic distribution of [1-13C] pyruvate, [1-13C] lactate and [1-13C] alanine in a mouse model of pancreatic cancer is demonstrated. This mouse was found to have a significant portion of high-grade pre-neoplastic lesions in the pancreas.
Pancreatic cancer (PCa) is one of the most aggressive solid malignancies, which can potentially be cured by surgery, making early diagnosis one of the best options for improving patient survival. Imaging plays an important role in the diagnostic algorithm for PCa,42 with the preferred imaging studies being DCE-CT or DCE-MRI43 and endoscopic ultrasonography, which may be useful in patients with equivocal CT/MRI findings.44 The role of 18F-FDG-PET remains unclear,43 however its major impact has been in the detection of small metastases45 and treatment monitoring. A recent study27 has shown that imaging with hyperpolarized [1-13C]pyruvate could make an impact in the early diagnosis of PCa in high-risk individuals, which are believed to comprise 36% of the cases of PCa.46–48 Imaging with hyperpolarized [1-13C]pyruvate could also potentially play a role in other clinical challenges in PCa, including differential diagnosis of PCa from chronic pancreatitis; treatment monitoring; and early anatomic detection of tumour recurrence.
Breast cancerDCE-MRI is the morphological reference imaging modality used in the assessment of breast cancer, with PET playing a growing role in the detection of metastatic lesions. Our increasing understanding of the molecular complexity and heterogeneity of breast tumours49 has led to the development of more individualised and flexible treatment strategies based on the patient's tumour type and their response to therapy. There is, therefore, an increasing need for new metrics that better characterise breast tumours, that can be used to assess their early response to treatment and that can be used to detect recurrence. Recent reports have shown that 18F-FDG-PET can play a role here,50–52 which suggests that metabolic imaging with hyperpolarized [1-13C]pyruvate may also have a role, with the added advantage that the absence of ionising radiation means that it should be possible to conduct multiple imaging exams, for example to screen for the presence of recurrence.
Brain tumoursDespite advances in treatment regimens in recent years, patient survival has not improved significantly, in particular for high-grade gliomas.53 Currently, DCE-MRI is the technique of choice for the clinical management of brain tumours; from initial diagnosis and biopsy-guided procedures to treatment planning and recurrence evaluation. Functional/biological characterisation of these tumours would be of value as it could help to tailor treatment at an early stage. Several studies have reported that metabolic imaging with PET can improve diagnosis. Indeed, application of 18F-FDG was shown to help in the differentiation of normal, low- and high-grade gliomas54,55; lesion delineation and postoperative detection of residual tumour.56 Despite the widespread clinical application of 18F-FDG, the use of amino acid tracers such as 11C methionine and 18F-fluoro-ethyl-tyrosine (18F-FET) have been reported to have improved detection sensitivity compared with 18F-FDG, particularly when assessing recurrence and differentiating it from treatment-induced changes.57,58 The potential advantage of using hyperpolarized 13C MRI in glioma evaluation is that it may allow co-registration of detailed anatomic and functional data in a radiation free manner, which would be of particular interest in paediatric patients. This may provide a more comprehensive characterisation of tumour localisation and heterogeneity and consequently improved tumour sampling, margin delineation for radiotherapy, early detection of treatment response and post-treatment detection of recurrence.
LymphomaMetabolic imaging with 18F-FDG-PET has long been used for staging and assessing treatment response in lymphoma patients.59 The current recommendation is that baseline and end of treatment 18F-FDG-PET images should be acquired. However, efforts are being made to include interim imaging, after either the second or fourth cycles of treatment, as a way for optimising outcome and minimising treatment toxicity. The greatest benefit of interim imaging will possibly lay in the potential to inform about “response-adapted therapy,” whereby treatment can be de-escalated in intensity in the setting of a satisfactory early response or escalated if early response is inadequate.60 With both approaches having shown promise in Hodgkin lymphoma,61–63 there are currently several ongoing clinical trials to further evaluate the value of response-adapted therapy based on findings of interim 18F-FDG PET/CT scans.64 Imaging, for example with hyperpolarized [1-13C]pyruvate, would have the potential to provide an improved radiation-free assessment of early treatment response, which might be of particular importance as a significant number of patients with lymphoma will be relatively young.
Future directionsClinical oncology practice relies increasingly on anatomic imaging at different stages of patient care. DNP has the potential to provide a new dimension and understanding of tumour biological behaviour, thus allowing a more personalised patient-centric approach. Despite its proven feasibility in humans and its significant potential in clinical oncology, DNP will still have to prove itself against established and emerging clinical techniques such as PET and demonstrate its added value in clinical practice.
Author contributionBoth authors listed, have made substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflicts of interestKB's lab has a research agreement with GE Healthcare (GEH) and holds patents on DNP technology with GEH.
Work in KMB's laboratory is supported by a Cancer Research UK Programme grant (17242) and the CRUK-EPSRC Imaging Centre in Cambridge and Manchester (16465). Clinical studies are funded by a Strategic Award from the Wellcome Trust (095962). E.M.S. was a recipient of a fellowship from the European Union Seventh Framework Programme (FP7/2007-2013) under the Marie Curie Initial Training Network METAFLUX (project number 264780). E.M.S. also acknowledges the educational support of the Programme for Advanced Medical Education from Calouste Gulbenkian Foundation, Champalimaud Foundation, Ministerio de Saude and Fundacao para a Ciencia e Tecnologia, Portugal.




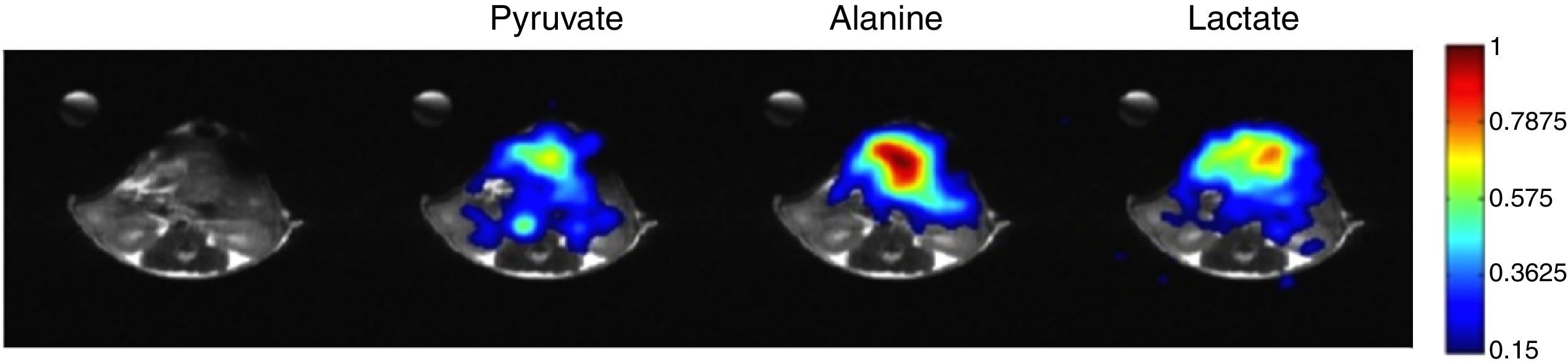
![13C chemical shift images acquired with a surface coil representing [1-13C]lactate, [1-13C]alanine and [1-13C]pyruvate peak intensities. Differential anatomic distribution of [1-13C] pyruvate, [1-13C] lactate and [1-13C] alanine in a mouse model of pancreatic cancer is demonstrated. This mouse was found to have a significant portion of high-grade pre-neoplastic lesions in the pancreas. 13C chemical shift images acquired with a surface coil representing [1-13C]lactate, [1-13C]alanine and [1-13C]pyruvate peak intensities. Differential anatomic distribution of [1-13C] pyruvate, [1-13C] lactate and [1-13C] alanine in a mouse model of pancreatic cancer is demonstrated. This mouse was found to have a significant portion of high-grade pre-neoplastic lesions in the pancreas.](https://static.elsevier.es/multimedia/24448664/0000000200000003/v1_201704300017/S2444866416301040/v1_201704300017/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)