The inability to maintain balance after stroke is an important risk factor for falling and relates to decreased potential for recovery. The vestibular system and gaze stability contribute respectively to postural stability and to maintain balance. Rehabilitation may be more effective with domiciliary training.
ObjectiveThis trial aims to verify if balance impairment after stroke improves with a domiciliary oculomotor and gaze stability training program.
MethodsIndividuals older than 60 years, discharged after suffering brain stroke with referral to the physiotherapy department, will be assessed for orthostatic balance. Patients with stroke diagnosis 3–15 months before recruitment, positive Romberg test and able to walk 3m alone are invited to participate in this randomized controlled trial. Participants will be allocated in two intervention groups through block randomization, either the current rehabilitation program or to a supplemental intervention focused on oculomotor and gaze stability exercises to be applied at home twice a day for three weeks. Primary outcome measures are the Motor Assessment Scale, Berg Balance Scale and Timed Up and Go Test. Trial registration: ClinicalTrials.gov (NCT02280980).
ResultsA minimum difference of four seconds in the TUG and a minimum difference of four points in BBS will be considered positive outcomes.
ConclusionsOculomotor and gaze stability exercises may be a promising complement to conventional physiotherapy intervention after brain stroke, improving the balance impairment.
Stroke is one of the major causes of long-term disability in the adult; balance deficits occurring after stroke are strongly associated with more severely impaired motor function and a decrease in recovery potential.1,2
In patients who have had strokes with inability to maintain balance, either in a static or in a dynamic way, it could be associated with the impairment to select reliable sensory information from different sources (visual, vestibular and somatosensory systems) in order to maintain postural stability using a correct motor pattern.3,4 Both postural imbalances after stroke and gait disorders are important risk factors for falls.5 The high incidence of falls in these patients is well documented in the literature, as well as its social and economic impact.6
The vestibular system contributes to postural stability and visual stabilization through the vestibulo-spinal reflex (VSR) and the vestibulo-ocular reflex (VOR), respectively.7
VOR is the first mechanism of gaze stability. During head movements, the VOR stabilizes gaze (eye position in space), generating eye movements of equal speed and opposite direction to the movement of the head8 to allow an adequate visual acuity,9 while the VSR contributes to maintain postural stability activating contraction of the antigravity muscles.7
Gaze stability is needed to coordinate the movements of the head, trunk and pelvis during walking.10 Individuals after stroke have been described to exhibit abnormal coordination of axial segments and pelvic rotations during head rotation, which can contribute to changes in balance during gait.11 The decrease in stability of the trunk and head after stroke also causes a lack of quality in visual information, which may cause impaired balance.11
Gaze stability exercises have been described to improve postural stability in healthy young adults,12 improve balance and subjective confidence to carry out the activities of daily life in a healthy elderly population13 and to decrease the perception of disability in individuals with unilateral vestibular deficit.14 It has been suggested that VOR adaptation exercises have influence on the alignment of the head, resulting on improvements in the overall perception of balance, expanding the limits of stability.15 The improvements achieved with these exercises in different clinical conditions were not associated with gender,16 age16,17 and time of onset of symptoms,17 therefore it may be assumed that they can be used both in chronic conditions and in the elderly.17
Oculomotor and gaze stability exercises are easy to learn, therefore, after supervised training, they can be performed at home,13 autonomously or with minimal supervision, as a complement to institution-based rehabilitation programs. Domiciliary training programs allow exercising at least twice a day, seven days a week, giving ground to quicker, more complete recovery.
This trial aims to verify if balance impairment after stroke improves with a domiciliary oculomotor and gaze stability training program for senior patients.
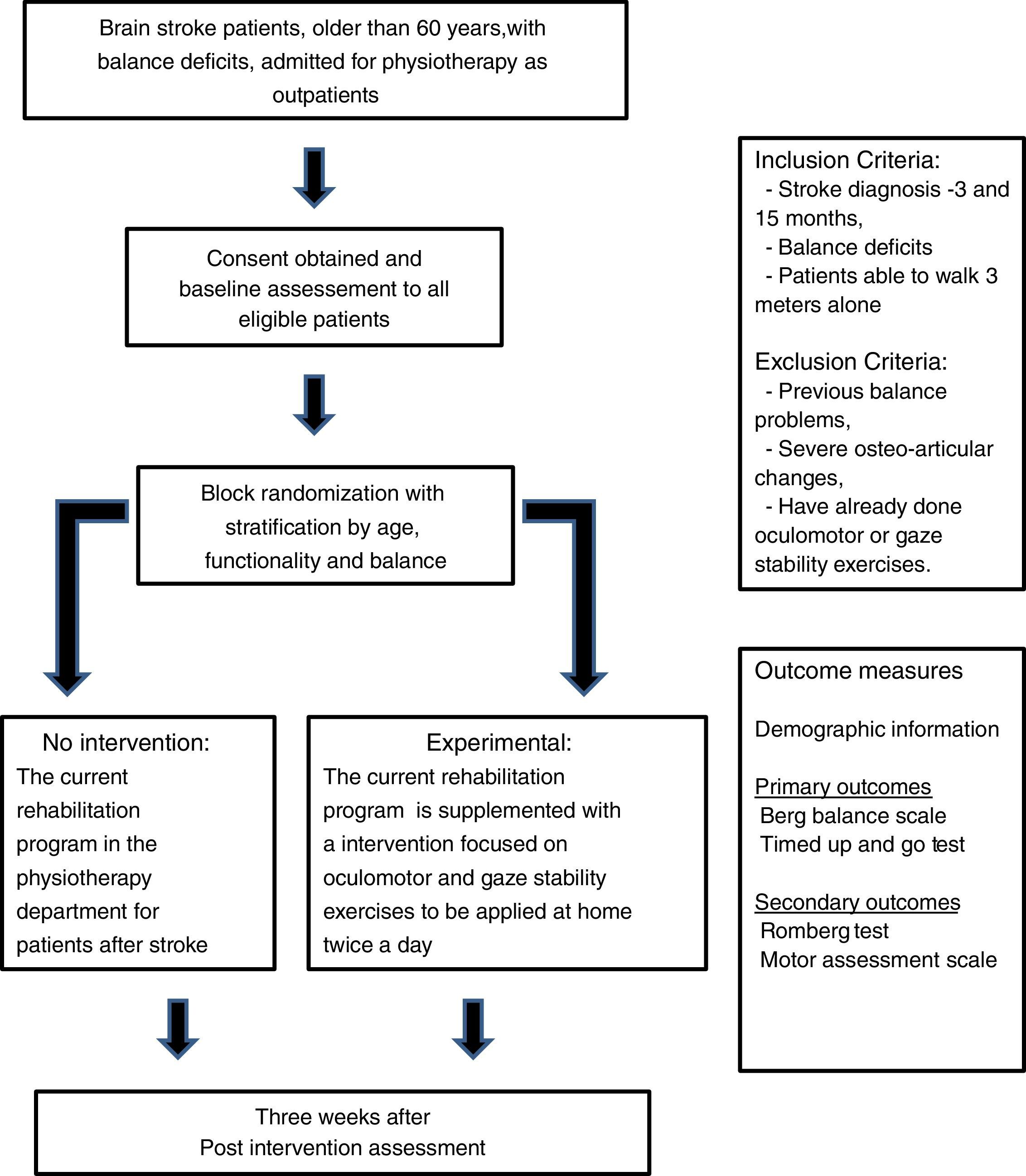
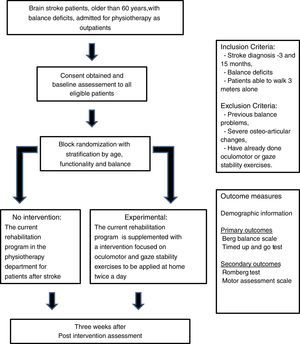
MethodsDesignNon-blinded, randomized controlled trial (RCT) (Fig. 1).
Patient populationIndividuals older than 60 years, discharged after suffering brain stroke with referral to the physiotherapy department outpatient clinic of a tertiary care hospital (Centro Hospitalar de Lisboa Central).
Outcome measuresPrimary outcome measures are:
- 1.
The variation in the Berg Balance Scale (BBS) score from baseline after suffering a brain stroke up to three weeks of intervention and its association to the home-based program of oculomotor and gaze stability exercises, and
- 2.
The variation in the Timed Up and Go Test (TUG) from baseline after suffering a brain stroke up to three weeks of intervention and its association to the home-based program of oculomotor and gaze stability exercises.
Individuals are eligible for the trial if they fulfill the following inclusion criteria:
- -
Brain stroke diagnosed 3–15 months prior to recruitment,
- -
Verified presence of impaired balance (positive Romberg test), and
- -
Ability to walk at least 3m alone with or without an assistive device.
Individuals are not eligible if:
- -
The balance problems are previous to the brain stroke,
- -
The ability to perform the proposed exercises is compromised by severe osteo-articular disease, or
- -
They had previous experience with oculomotor or gaze stability exercises.
After the initial assessment, participants will be allocated in two intervention groups through block randomization with stratification by age, functionality and balance. Three age groups will be considered: 60–69 years, 70–79 years and ≥80 years. Patients will be stratified by their functionality into three categories, according to the score of the Motor Assessment Scale (MAS): major dependence (score below 16), moderate dependence (score between 17 and 32) and minor dependence (score over 33), and by their balance into two categories, according to the predictive cut-off points for falling using TUG18 and BBS19: no risk of falling (TUG<14s and BBS>45) or with risk of falling (TUG>14 and/or BBS<45).
Sample sizeThe sample size was estimated considering the ability to identify (power 90% and confidence 90%) either a minimum increase of four points in BBS20 or minimum decrease of four seconds in TUG.20,21 The estimated minimum sample size to detect four seconds of difference in the TUG in individuals with the target population characteristics is 18 elements. The estimated minimum sample size to detect a difference of 4 points in BBS in individuals with the target population characteristics is 66 elements, thus this will be the estimated target sample size.
Study proceduresAfter checking for eligibility criteria, the patients will be invited to participate and informed, written consent will be obtained.
Participants will have a baseline assessment with MAS (to access the level of dependence), BBS and TUG (to access balance). Furthermore, demographic and clinic information will be collected by interview and confirmed by consulting the previous clinical records (when available), including the date of the stroke, location, laterality and etiology, and participants will be asked about previous balance problems, treatments with oculomotor or gaze stability exercises, severe osteo-articular problems, gait ability, number of falls after stroke, and present therapies.
The rehabilitation program for stroke patients, in this unit, is customized according to the patient problems and based on the professional's clinical reasoning supported in the knowledge of neurophysiology, motor control, biomechanics and motor learning theories,22 using a mixture of components from several different approaches.23
Participants will be randomly assigned to either the usual rehabilitation program only or to the program with a supplemental intervention, to be applied at home for three weeks, as used in the study of Morimoto and colleagues.12
Participants in the supplemental intervention group will be taught a set on oculomotor and gaze stability exercises (Table 1) and will receive a leaflet and a logbook. When the participants have difficulties in learning or performing the exercises by themselves, a caregiver will be required to collaborate.
Description of the oculomotor and gaze stability exercises (based on Morimoto and colleagues12).
| The home protocol consists in eight different oculomotor and gaze stability exercises | |
| Exercise 1 | Moving the eyes horizontally between two stationary targets while keeping the head still – saccadic eye movement exercises. |
| Exercise 2 | Moving the eyes vertically between two stationary targets while keeping the head still – saccadic eye movement exercises. |
| Exercise 3 | Moving the target horizontally and tracking it with the eyes while keeping the head still – smooth pursuit exercises. |
| Exercise 4 | Moving the target vertically and tracking it with the eyes while keeping the head still – smooth pursuit exercises. |
| Exercise 5 | Moving the head horizontally while keeping the look on a stationary target – adaptation exercises. |
| Exercise 6 | Moving the head vertically while keeping the look on a stationary target – adaptation exercises. |
| Exercise 7 | Moving the head and target in opposite directions horizontally while tracking the target with the eyes – adaptation exercises. |
| Exercise 8 | Moving the head and target in opposite directions vertically while tracking the target with the eyes – adaptation exercises. |
| Participants should perform the exercises at home, standing, twice a day for three weeks. Each exercise should be repeated 10 times. | |
| Participants are instructed to move the target, or their head, slowly while maintaining clear focus on the target during the exercises. | |
| If the participants feel any kind of imbalance or dizziness sensation, during the exercises, they should make a small pause and restart when possible. | |
| All exercises are explained and made with participants to ensure the correct performance. If necessary, a third person will help the patient for correct and safe performance. | |
The supplemental exercises will be reviewed every week with the participants to check the compliance with the home program, to clarify doubts and to register difficulties or possible adverse effects.
After three weeks, every participant will be submitted to a balance assessment (BBS and TUG) and will be asked about the number of falls that occurred. The participants in the supplemental intervention group will be asked to return the logbook to the investigators.
All the assessments, the training for the exercises and their periodical review are performed by one of the two physiotherapists responsible for the trial.
Romberg test – It is a static balance test,24 performed on a stable surface, in which the patient stands with their feet together, first with the eyes open and then with the eyes closed; the test is repeated on an unstable surface (balance pad with 60mm thick) under the same conditions. The test is timed, considering the time until the patient either moves a foot from the initial position, opens his eyes or reaches the maximum time of 30s. Any of the conditions before 30s will be considered loss of balance and a positive Romberg test.25 Compensatory movements of the upper limbs or trunk are accepted.
Berg Balance Scale (BBS)26,27 – It is an instrument to evaluate balance by assessing the performance on 14 functional tasks in older people with impairment. The total score ranges from 0 to 56 points. A score lower than 45 points is considered as risk of falling.19 In a systematic review of the assessment of balance it was found that most studies used the BBS and found strong evidence that this scale is sensitive to balance disorders in acute stroke patients and in the chronic phase of stroke in patients with low initial BBS score.2
Timed Up and Go Test (TUG) – It is a simple test used to assess mobility and requires both static and dynamic balance. Several studies use TUG as an indicator for the risk of falling.18,28 A value greater than 14s is considered predictive of risk of falling in elderly community.18
Motor Assessment Scale (MAS) – It is a performance-based scale developed to assess everyday motor function in patients with stroke.29 It consists of 8 items corresponding to different areas of motor function, each item is scored from 0 to 6, and the maximum score represents optimal performance.
Data analysisData will be analyzed using descriptive statistics and statistical inference (univariable and bivariable), both as for intention to treat and as per protocol. The participants who are not able to learn the exercises of the home program; those with lack of adherence (less than 50% of the proposed plan) and those that interrupt the usual rehabilitation program for more than one week for any reason will be identified and excluded from the per protocol analysis. The multivariable analysis will take into account the time lapse from stroke to intervention and the occurrence of previous known strokes, as potential confounders.
Ethical considerations and registrationInformed, written consent will be obtained following screening for eligibility criteria. The protocol was approved by the Ethical Committee of Centro Hospitalar de Lisboa Central and was registered at ClinicalTrials.gov (NCT02280980).
Expected resultsA minimum difference of four seconds in the TUG and a minimum difference of 4 points in BBS will be considered positive outcomes.
For the per protocol analysis, the difference of both BBS and TUG between baseline and the final assessment will be compared using paired samples tests. Risk ratios for positive outcomes with 95% confidence intervals will be estimated. Regression models will be used to explore factors that affect the odds for success.
For the intention to treat analysis every patient will be considered; those that do not comply and those that do not achieve the minimum differences considered for BBS or TUG as positive outcomes will be considered as failures. Hazard ratios for positive outcomes with 95% confidence intervals will be estimated. Mixed models of structured equations will be used for analysis of longitudinal data to explore factors that affect the odds for success.
Final considerationsThe effectiveness oculomotor and gaze stability exercises in improving postural stability and balance has been proven by several studies in healthy individuals,12 patients with multiple sclerosis30 and with vestibular disorders.31–33 These exercises may prove to be a promising approach to be included as a complement in the physiotherapy intervention after stroke, when balance deficit is present. This trial aims to verify if balance impairment after stroke improves with a domiciliary oculomotor and gaze stability training program for senior patients. The trial may be affected by some confounders, such as the topography of the stroke, associated new and previous impairments, different recovery potential and the customization of the current rehabilitation program to specific patient needs.
FundingNone declared.
Authors’ contributionsAll authors participated in developing the design of the study and contributed to and critically appraised the manuscript. The authors have given final approval of the version to be published and they confirm that there are no other persons who satisfied the criteria for authorship.
Conflict of interestThe authors declare no conflicts of interest.
We acknowledge the staff from the Department of Physical Medicine and Rehabilitation at Curry Cabral Hospital (Centro Hospitalar de Lisboa Central). No external funding was granted to this institutional clinical trial.