Myasthenia Gravis is a humoral autoimmune disorder affecting the neuromuscular junction. Its treatment is based on immunosuppressive agents. Rituximab has shown efficacy in refractory and severe Myasthenia Gravis. We evaluate the potential pharmacoeconomic and quality of life benefits of its use.
MethodsA retrospective analysis of Myasthenia Gravis patients treated with Rituximab was performed. Clinical charts were reviewed and scales for assessment of quality of life were applied. Health care costs were estimated based on the average of each treatment and daily charge of hospitalization.
ResultsSix patients were treated. Rituximab use lead to the reduction of relapses and to a lesser use of immunosuppressive agents. An overall decrease in healthcare costs after treatment was observed along with an evident clinical improvement.
DiscussionRituximab is a clinical effective treatment for B cell-related diseases like MG and seems to be a cost–saving intervention. Its use is associated with a decrease in the need for other immunosuppressive treatments whilst improving quality of life and reducing health costs.
Myasthenia Gravis (MG) is a rare autoimmune disorder affecting the neuromuscular junction caused by antibodies (Abs) against postsynaptic membrane proteins, thus preventing an effective neurotransmission at the synaptic terminal.
The most important membrane antigen is the muscle acetylcholine receptor (AchR) as AchR-Abs are present in the serum of 85–90% MG patients. About 40% of the remainder AchR-negative patients have Abs directed against the muscle-specific tyrosine-kinase (MuSK). In some patients with a generalized form of MG, antibodies against low-density lipoprotein receptor-related protein 4 (LRP4) may be present. MuSK and LRP4 are not directly involved in the neuromuscular transmission, but in the end-plate maturation. The clinical phenotype of anti-MuSK is associated with a poorer response to standard therapies compared to AChR-positive patients, whilst the LRP4 subset resembles closely that of anti-AChR-positive MG. Recently a new epitope antibody was identified in patients with MG, Anti-agrin.1,2 Even with 4 antibodies identified, a small proportion of patients remain seronegative.
The goal of MG treatment is to induce and then maintain disease remission. Current treatment options include acetylcholinesterase inhibitors, short-term immune therapies (plasmapheresis or intravenous immunoglobulin), and long-term treatment with corticosteroids and classic immunosuppressive agents, namely azathioprine, mycophenolate mofetil and cyclosporine, among others.3
Treatment with immunosuppressors has reduced mortality and significantly improved the prognosis of these patients. However, a subset of patients has refractory disease or requires high doses of immunosuppressive agents with multiple side effects.3
Recently, several authors have described the role and efficacy of Rituximab (RTX), a chimeric mouse/human monoclonal antibody that targets CD20 B lymphocytes in the treatment of drug-resistant MG.4–7
RTX was first used for the treatment of haematological malignancies, mainly non-Hodgkin's lymphoma and in this context was found useful for patients with lymphoma-associated MG.8,9 It is also used for the treatment of several autoimmune diseases in which B cells have a predominant pathogenic role such as systemic lupus erythematosus and rheumatoid arthritis.10
MG is an “antibody associated disease” with B cells having an important role in its pathogenesis. Therefore, the use of RTX in MG has been increasingly suggested, but reports are still restricted to minor series or individual cases.1
To our knowledge, no studies have described the impact of this new therapeutic approach in the quality of life, nor have been determined its economic implications. Having different therapeutic options makes it essential to establish the costs and benefits of this new treatment in comparison with the former available options. The quality-adjusted life year (QALY) is an outcome measure that merges quality and duration of life and is crucial to cost–effectiveness analysis.11
We performed a cost–utility analysis regarding the treatment of six MG patients with RTX, taking into account major aspects such as clinical and serologic characteristics, objective evaluation of quality of life and overall treatment costs.
Material and methodsThe use of rituximab (RTX) in MG in our centre is confined to patients with refractory4 and/or severe1 MG or with a concomitant autoimmune disease in which rituximab therapy has proven efficacy such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA). Refractory patients are defined when they cannot lower their steroid therapy without clinical relapse, are not clinically controlled on their immunotherapy regimen, or have severe side effects from immunosuppressive treatments. Severe MG was defined as a classification of MGFA≥IIIb.
Our protocol of administration consists of a course of treatment that is composed of 2 infusions of 1000mg given 15 days apart. Retreatment is decided in a multidisciplinary team (neurology and internal medicine specialists, with experience in autoimmune diseases) and based on disease activity, CD19 lymphocyte plasma count and serum immunoglobulin levels, with a minimum interval between infusions of 4 months.
Six patients with generalized MG were treated with Rituximab, since February 2010, and followed in the Neurology clinic and Autoimmune Diseases Unit of our Hospital. We performed a retrospective study and collected data from the clinical records of those patients since the beginning of their follow up in our hospital until September 2015.
The patients fulfilled the diagnostic criteria for MG on the basis of clinical history, neurological examination and evidence of electrophysiologic neuromuscular transmission defect.
We assessed clinical data, quality of life and economic costs before and after treatment with RTX. Clinical data included (a) prednisone dose, (b) number of imunossupressors, (c) IVIg and plasmapheresis treatments used for myasthenic crisis, (d) Myasthenia gravis composite scale (MGCS),12 (e) Myasthenia Gravis Post interventional status (MGFA-PIS) 15,13 and (f) safety data/side effects. We used paired t-test analysis to evaluate differences; results were considered significant when p<0.05.
Quality of life was assessed applying a generic (EQ-5D-3L, Portuguese version, which includes a questionnaire and a visual analogic scale),14 and a disease specific (MG-QOL 15) quality of life instrument.15
EQ-5D is an instrument developed by EuroQol which allows the measure of health-related quality of life to be used in cost–utility economic evaluations. It defines health status in terms of domains or dimensions, which are convertible to a numeric value associated with the health status described (health utilities). Health utilities can be used to compare improvement or decrease in quality of life status, and using a time factor (for example the time between two evaluations) it can be used to calculate QALY's. The higher the numeric value, the higher is the quality of life measured with EQ-5D. MGQOL-15 is a disease specific MG quality of life measurement that uses 15 questions to evaluate the 4 domains, namely mobility (9 items); MG symptoms (3 items); general contentment (1 item); and emotional well being (2 items). The higher the total score, the lower is the quality of life related to MG.
These instruments were applied after the collection of the clinical information. Patients were asked to complete the above-mentioned questionnaires referring to the moment before starting treatment with RTX and to their current status. To assess the convergent validity between values obtained from the general quality of life instrument (validated to Portuguese language (EQ-5D), but not for MG), and the quality of life instrument specifically developed and validated to Myasthenia Gravis (MG-QOL-15), we used Pearson correlation test.
Pharmacoeconomic methodsHealth state utilities were transformed into quality adjusted life years (QALYs) by using the time of follow up, and assuming a linear evolution over time as commonly suggested.16
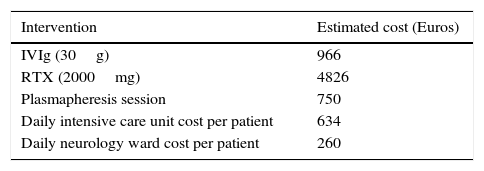
Estimated healthcare costs were calculated based on the average cost of RTX, plasmapheresis session, human immunoglobulin, the daily charge of hospitalization on a Neurology ward and in an Intensive care unit. All costs are expressed in Euros (€). Appendix 1 can be visualized for further detail.
The cost–utility analysis was performed making a direct comparison between the cost/patient in the year before and the year after RTX treatment, and using as utility marker the calculated QALY/patient/year, value.
Statistics were performed using IBM SPSS Statistics version 21.
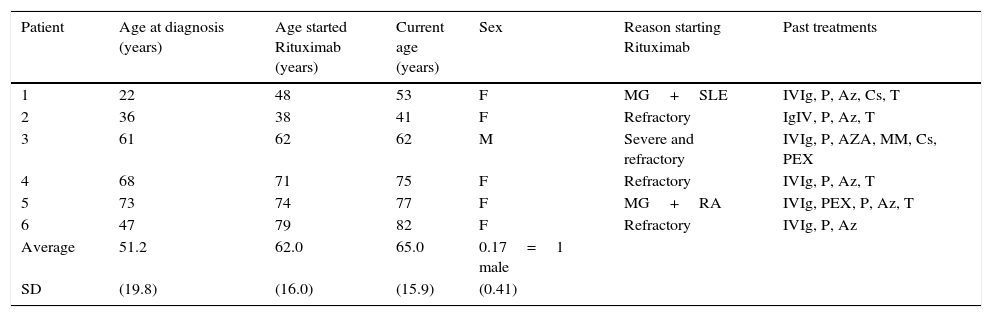
ResultsA total of 6 patients (5 females and 1 male) with a mean age of 65 years (standard deviation (SD) 15.9 years) were studied. The mean age of disease onset was 51.2 years (SD 19.8 years), and time between diagnosis and treatment with rituximab was 10.8 years (SD 12.8 years). Patients mean follow up time after starting rituximab was 39 months (ranging from 11 to 67 months).
All patients were diagnosed with generalized MG, 4 of them were AChR-IgG+ and 2 were seronegative. The majority of patients started rituximab due to refractory MG, yet 2 patients initiated treatment in order to control a concurrent autoimmune disease (SLE and RA).
Clinical response to treatmentA summary of the clinical response is expressed in Tables 1 and 2.
Patients clinical and demographic characteristics.
| Patient | Age at diagnosis (years) | Age started Rituximab (years) | Current age (years) | Sex | Reason starting Rituximab | Past treatments |
|---|---|---|---|---|---|---|
| 1 | 22 | 48 | 53 | F | MG+SLE | IVIg, P, Az, Cs, T |
| 2 | 36 | 38 | 41 | F | Refractory | IgIV, P, Az, T |
| 3 | 61 | 62 | 62 | M | Severe and refractory | IVIg, P, AZA, MM, Cs, PEX |
| 4 | 68 | 71 | 75 | F | Refractory | IVIg, P, Az, T |
| 5 | 73 | 74 | 77 | F | MG+RA | IVIg, PEX, P, Az, T |
| 6 | 47 | 79 | 82 | F | Refractory | IVIg, P, Az |
| Average | 51.2 | 62.0 | 65.0 | 0.17=1 male | ||
| SD | (19.8) | (16.0) | (15.9) | (0.41) |
AChR, acetylcholine receptor; Az, azathioprine; Cs, cyclosporine; IVIg, intravenous immunoglobulin; MM, mycophenolate mofetil; P, prednisone; PEX, plasmapheresis; RA, rheumatoid arthritis; SD, standard deviation; SLE, systemic lupus erythematosus, T, thymectomy.
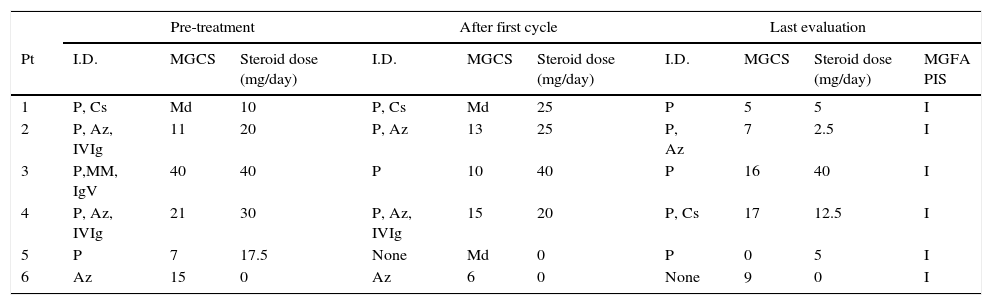
Summary of clinical response to treatment with rituximab.
| Pre-treatment | After first cycle | Last evaluation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | I.D. | MGCS | Steroid dose (mg/day) | I.D. | MGCS | Steroid dose (mg/day) | I.D. | MGCS | Steroid dose (mg/day) | MGFA PIS |
| 1 | P, Cs | Md | 10 | P, Cs | Md | 25 | P | 5 | 5 | I |
| 2 | P, Az, IVIg | 11 | 20 | P, Az | 13 | 25 | P, Az | 7 | 2.5 | I |
| 3 | P,MM, IgV | 40 | 40 | P | 10 | 40 | P | 16 | 40 | I |
| 4 | P, Az, IVIg | 21 | 30 | P, Az, IVIg | 15 | 20 | P, Cs | 17 | 12.5 | I |
| 5 | P | 7 | 17.5 | None | Md | 0 | P | 0 | 5 | I |
| 6 | Az | 15 | 0 | Az | 6 | 0 | None | 9 | 0 | I |
Az, azathioprine; Cs, cyclosporine; IVIg, intravenous immunoglobulin; I, improved; I.D., immunosuppressive drugs; Md, missing data; MM, mycophenolate mofetil; Pt., patient; P, prednisone; PIS, post-interventional status; U, unchanged; MGCS, MG composite scale.
We observed a decrease in the MGCS mean score after the first cycle of RTX and an even more relevant decrease at the final evaluation, 36% and 53% (p=0.028) respectively.
All our 6 patients were on treatment with several immunosuppressors (average of 2.2 drugs). After the first cycle of RTX therapy that number was reduced in 33%, to a mean of 1.5 drugs per patient and had a further reduction of 47% to a mean of 1.2 (p=0.012) at the final evaluation.
Five out of 6 patients were on oral corticosteroids. None of them was able to completely taper off prednisone after starting rituximab treatment; however, there was a significant reduction of 53%, from an average dose of 23.5mg/day to 13mg/day (p=0.047).
The number of treatments with PEX (cycle of 5 sessions) or IVIg (cycle of 5 treatments) in the year before and after rituximab treatment, were considered as surrogate markers for MG acute exacerbations. A reduction of 83% on average treatments (p=0.027) was observed, with only 1 patient being treated with IVIg in the year following the beginning of RTX treatment. We also found an overall significant decrease of short-term treatments after RTX, (90%, p=0.003).
After Rituximab treatment all of our patients were classified as “Improved” (MGFA-PIS). This level is obtained as a result of clinical improvement and/or reduction of the MG medications.
Side effectsIn our series, two patients experienced major secondary effects associated with RTX, namely a case of sustained hypogammaglobulinemia (patient 4) and a macrophage activation syndrome (patient 2). The latter was reported as a possible side effect of RTX, although a clear clinical causation was not established. We underline the fact that both patients with major side effects to RTX were still under two immunossupressors.
Two patients experienced minor side effects: patient 5 had recurrent respiratory tract infections which did not require hospitalization, and patient 2 had an infusion reaction (flushing, rigours and hypotension) during the first administration but subsided during the following administrations of RTX.
We monitored CD19 plasma levels regularly, and found the repopulation of these lymphocytes was associated with clinical worsening which might be used as an useful clinical marker.
Quality of lifeIn order to determine an effect of treatment on quality of life, we calculated a mean variation (XΔ) of the 3 scores (EQ-5D questionnaire score, EQ-5D Visual Analogic scale and MG-QOL 15).
The XΔ EQ-5D score was +0.492, the XΔ EQ-5D VAS (visual analogic scale) was +48.3 and the XΔ MG-QOL15 was −20. These values showed a positive tendency towards an improvement in the quality of life after treatment.
There was a significant correlation between the individual scores of the 3 instruments. The positive correlation between the XΔ EQ-5D question score and the XΔ VAS score (r=+0.936, p=0.006) confirms the internal validity of the EQ-5D instrument.
We also observed a significant correlation between the XΔ EQ-5Q question score and the XΔ MG-QOL 15, the XΔ EQ-5D VAS and the XΔ MG QOL 15 (r=−0.979, p=0.001 and r=−0.929, p=0.007 respectively). These findings suggest that there was coherence between the results provided by the two different scores, confirming the convergent validity.
There was a positive variation in QALY during the time of treatment corresponding to +1.76/patient, which means that quality-life adjusted years were gained.
ΔEQ-5D multiplied by the factor one (year) is approximately the same as the QALY gained during one year of treatment with Rituximab, and obtained the value of 0.492. A detailed analysis considering health utilities (EQ-5D variation), can be visualized in Table 3.
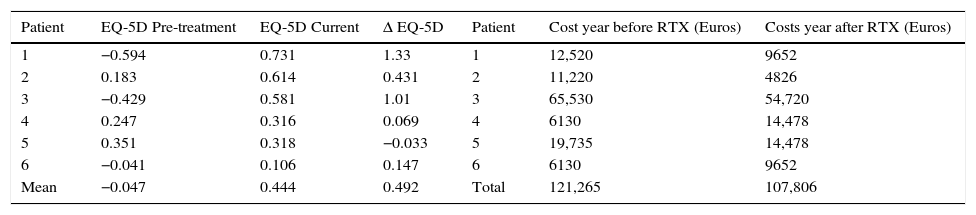
Cost–utility analysis per patient.
| Patient | EQ-5D Pre-treatment | EQ-5D Current | Δ EQ-5D | Patient | Cost year before RTX (Euros) | Costs year after RTX (Euros) |
|---|---|---|---|---|---|---|
| 1 | −0.594 | 0.731 | 1.33 | 1 | 12,520 | 9652 |
| 2 | 0.183 | 0.614 | 0.431 | 2 | 11,220 | 4826 |
| 3 | −0.429 | 0.581 | 1.01 | 3 | 65,530 | 54,720 |
| 4 | 0.247 | 0.316 | 0.069 | 4 | 6130 | 14,478 |
| 5 | 0.351 | 0.318 | −0.033 | 5 | 19,735 | 14,478 |
| 6 | −0.041 | 0.106 | 0.147 | 6 | 6130 | 9652 |
| Mean | −0.047 | 0.444 | 0.492 | Total | 121,265 | 107,806 |
Regarding the evaluation of costs with RTX, we calculated the total expenses of the one year before and the one after initiating treatment. This option allowed for a direct comparison of the yearly cost of MG treatment before and after RTX. The total cost in the year previous to RTX treatment was 121.265€ and in the year after was 107.806€. There was a reduction in costs of 2243€/patient/1st year.
Additionally, we calculated the yearly cost/patient using the total follow up time (39 months=3.25 years) when treating these patients with Rituximab. It corresponds to 11.677€/patient/year. The cost per patient during the first year of treatment with rituximab was higher than the one in the following years, which can be inferred calculating the average cost/patient/year during follow up, that corresponds to 8.881€/patient/year, compared to the 1st year cost 17.967€. This decrease in costs compared to the first year of treatment derives essentially from a decreased number of Rituximab infusions in the follow up period (mean of 1st year infusions/patient=2.16; mean follow up infusions/patient=1.4).
Cost–utility analysisWhen comparing healthcare costs in the year before and the one after RTX, we observed an overall decrease (−2243€ per patient), which was associated with an increase in QALYs (0.492). Hence, treatment with Rituximab was a cost–saving intervention in our patients, at least during the first year.
DiscussionIn this analysis of 6 patients with refractory and non-refractory generalized MG we demonstrated a sustained clinical response and a reduction in concomitant immunosuppressive medication after starting treatment with RTX. We also observed an improvement in general quality of life during treatment with RTX when compared to a similar period of time immediately before starting treatment with this drug. We showed that in a one-year analysis, RTX treatment was a cost–saving intervention, and the costs associated to this treatment decreased further during follow up.
Quality of life and healthcare expenditures of this therapeutic option for MG has not been reported before in the existing observational/small case series. This topic is very important when considering the limited financial resources of any health system and the potential unlimited costs due to the multiplicity of therapeutic options.
The positive clinical response observed in our patients is in agreement with other series (6, 7), and the recent meta-analysis by Iorio and colleagues, where high overall response rates are reported show that these results do not differ significantly between different serologic groups.
In our cohort, we could not reproduce the dramatic response (clinical and pharmacologic remission) reported by others, in patients with anti-MUSK antibodies.17,18
Our follow up time was from 11 to 67 months, which suggests that RTX may have a sustained response after several months or even years of treatment. We additionally noticed a decrease in the need of RTX infusions to control the disease during the follow up. We postulate that immunomodulation granted by RTX persists in time and is cumulative, and recommend that the intervals between infusions be tailored according to MG symptoms and signs (objectively measured), and not according to a fixed timespan.
We found one definite and one possible major side effect to RTX, namely a sustained hypogammaglobulinemia and a macrophage activation syndrome, which led to temporary discontinuation of treatment in these patients. They both occurred on double therapy which highlights the importance of reducing or discontinue all other immunosuppressors if possible. We had no cases of progressive multifocal leukoencephalopathy.
Our study has limitations: the population size is small and there is no control group; it is retrospective and therefore some data regarding the clinical response was qualitative, namely the MGFA-PIS. Nevertheless, the systematic use of a quantitative instrument (MGCS) in our patients, allowed an objective assessment of the clinical variations during the treatment with RTX.
The assessment of the quality of life was performed with two different instruments (EQ-5D and MG-QOL 15). Although they were used in a retrospective fashion (which introduce a memory bias in our data analysis), their results suggest that the QALY's calculation was indeed valid and truly represented the clinical implications of MG in the quality of life of the patients.
RTX is a valuable therapeutic option for patients with MG who are poorly controlled or require high doses of immunosuppressors or when there are other concomitant autoimmune diseases, in which there is an established benefit for targeting CD20 lymphocytes. We underline this particular aspect in our series, as two of our patients had concomitant autoimmune diseases and these associations are not rare.
Although our results in terms of clinical response, quality of life and cost–utility, favour the use of rituximab, they would need further investigation in a prospective, controlled manner. Aspects that may be addressed in further studies would include: (1) Effective dose of Rituximab, as the current protocols are adapted from other diseases and there is a report of efficacy with a dose of 1000mg per cycle.19 This would decrease even more the cost of RTX therapy in MG and potentially reduce the side effects; (2) Quality of life as one of the primary endpoints; (3) Cost–utility analysis using objective instruments such as the EQ-5D (which has a correlation MG-QOL 15) to find QALY's.
Conflict of interestThe authors declare no conflict of interest.
We are grateful to Bruno Martins M. Ec. for his contribution reviewing our work.