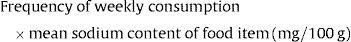Helicobacter pylori infection is mainly acquired during childhood and is associated with an increased risk of developing gastric cancer. High amounts of sodium intake can lead to the onset of pre-malignant lesions contributing to the process of carcinogenesis, and potentiate the effect of H. pylori infection. This study aimed to evaluate the exposure to sodium in children until 4 years of age and to quantify its association with H. pylori infection.
MethodsThis study includes 503 children from the cohort Generation XXI, recruited after childbirth and re-evaluated at 6 months and at 4 years of age. Information about socio-demographic characteristics, food intake after birth and status of H. pylori infection (assessed by serum ELISA) was collected. Scores of sodium exposure were calculated using the consumption of milk and other food items (evaluated at 6 months), and food items with the highest contribution to sodium intake and sodium intake (evaluated at 4 years). Logistic regression models were used to compute adjusted odds ratio (OR) and respective 95% confidence intervals (CI).
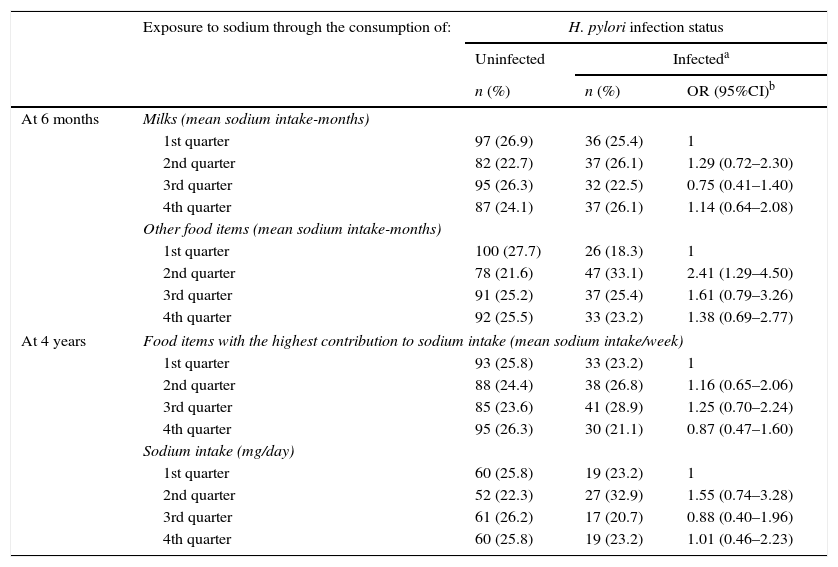
ResultsWe found that 28.2% of children were infected with H. pylori at 4 years of age, with a daily sodium intake that exceeded World Health Organization recommendations in 26%. No statistically significant association between sodium intake and H. pylori infection was observed, with the exception of the 2nd quarter in the score concerning consumption of “other food items” in the assessment at 6 months of age (OR=2.41; 95%CI: 1.29–4.50).
ConclusionNo association between sodium intake and H. pylori infection was found; however, to the best of our knowledge, the present epidemiologic study is the first to test the influence of sodium intake in H. pylori infection in children.
Gastric cancer is the third most common cause of cancer deaths worldwide.1 In Portugal, gastric cancer mortality has been decreasing since the 1970s, though with large regional differences.2 The high prevalence of Helicobacter pylori, a major risk factor for gastric cancer, at young ages is associated with a higher mortality due to this type of cancer compared with cases for whom the infection was acquired later in life.3
Although dietary sodium intake is independently associated with an increased gastric cancer risk,4 a significant positive interaction may exist between this factor and H. pylori infection contributing to the risk of gastric cancer.5 The biological plausibility for such a synergism has been demonstrated in animal studies.6,7 Excessive sodium intake may increase H. pylori colonization and exacerbate gastritis, enhancing the carcinogenic process,6 and a dose-response relationship was even demonstrated in experimental models.7 However, no epidemiologic study has tested the influence of sodium intake in H. pylori infection. Demonstrating the existence of a causal relationship between sodium intake and H. pylori infection would allow for the identification of potential targets for intervention strategies for the prevention of infection by H. pylori, resulting in a decrease in the future burden of gastric cancer.
This study aimed to evaluate the exposure to sodium in children until 4 years of age, through data collected since birth, and to quantify its association with H. pylori infection in the early stages of life.
MethodsStudy participants and data collectionThe present study was carried out within the cohort Generation XXI (G21). The cohort comprises 8647 children, born between April 2005 and August 2006 in the Porto Metropolitan Region.8,9 Recruitment was conducted at five public maternity units, responsible for 95% of the deliveries in the region in that moment, and 91.4% of the eligible mothers accepted to participate.
All phases of the study complied with the Ethical Principles for Medical Research Involving Human Subjects expressed in the Declaration of Helsinki. The study was approved by the University of Porto Medical School/S. João Hospital Centre Ethics Committee and by the Portuguese Authority of Data Protection. Written informed consent was obtained from all participants.
RecruitmentParticipants were face-to-face interviewed by trained interviewers between 24 and 72h after delivery. Data were collected using structured questionnaires on maternal socio-demographics, obstetric and gynaecologic history, planning and occurrence of the current pregnancy, prenatal care, lifestyle before and during the pregnancy. Pregnancy complications, delivery and newborn-related data were retrieved from the medical records by the same interviewers using standard forms. They also performed more detailed measurements of the newborns as well as of their parents (i.e., weight, height and head circumference) using standardized methods. Whenever possible, venous blood samples were collected from the parents, as well as an umbilical cord sample of the children. Serum samples were stored at −80°C until analysis.
Re-evaluation at 6 months of ageTrained interviewers collected information about each child through a standardized questionnaire, including socio-demographic and clinical data, and food consumption. Besides information on breastfeeding practices, the consumption of other types of milk was also registered. Additionally, data on the consumption of other food items was collected, namely, water, tea, tisanes, barley, juices, yogurts, fruit, cereal and gluten free cereal, bread, cookies, eggs, vegetable soup, potatoes, meat, fish, salt and sugar, as well as the age at which these food items started to be consumed by the child.
Re-evaluation at 4 years of ageBetween April 2009 and August 2011, a follow-up evaluation of the entire cohort was performed. Most children were evaluated in a face-to-face interview, a physical examination was also performed and a blood sample was collected from the children and their mothers, whenever possible. Parents answered a structured questionnaire to gather data on socio-demographic characteristics, physical activity, dietary habits, sleep features and healthcare use. Information on the main caregiver for the child since birth was also collected. Furthermore, a food frequency questionnaire was applied, and the parents were asked to fill a 3-day food diary at home (two week days and one day of the weekend). Conversion of food items into nutrients was performed using the database Food Processor Plus software (1997; ESHA Research, Salem, Oregon, USA), which has been adapted to traditional Portuguese food and dishes presented in the Portuguese table of food composition.10
Children whose parents had provided a blood sample at the baseline evaluation were eligible for this study. Mothers from the children included in this study (n=503) were older and more educated than the total number of mothers recruited in G21.
Scores of exposure to sodiumTo estimate the amount of sodium intake at 6 months of age through milk, data regarding the sodium content per 100ml of product for each specific milk consumed by the child since birth, as reported by the parents, was retrieved. The different types of milk, its commercial designation and the age at which the child started and ended, if applicable, its consumption were also taken into account. The mean sodium content of the milks reported to be consumed by the children was estimated to be 20mg/100ml. This value was used as a reference to calculate exposure to sodium through milk consumption. For each milk, we also computed the time of exposure by subtracting the end date to the start date of consumption. For children consuming more than one type of milk since birth, the different parcels were summed to build a single score.
Exposure to sodium through milk consumption at 6 months of age (mean sodium intake in months)Regarding the other food items consumed at 6 months of age, information concerning their sodium content was retrieved from the Portuguese table of food composition,11 considering the mean sodium content in each food item per 100g of product. To build the score of exposure to sodium for each food item, the time of exposure to that specific food item (difference between end date and start date of consumption reported by the parents) in months was multiplied by the value obtained for the mean sodium content per 100g.
Exposure to sodium through consumption of other food items at 6 months of age (mean sodium intake in months)Daily sodium intake was evaluated through 3-day food diaries at 4 years of age. These diaries allowed for the identification of food items with the highest contribution to sodium intake, such as: vegetable soup; dairy products; meat, fish and eggs; cereal and potatoes; cookies and biscuits; savory snacks; ham, bacon and sausages; fruits and vegetables; cakes and sweets.12 These food items were also part of the food frequency questionnaire applied at 4 years of age, and we evaluated the frequency of consumption for each one. To build the score of sodium exposure for the food items with the highest contribution to sodium intake, the number of times that the food item was consumed per week was multiplied by the mean sodium content of that specific item.
Exposure to sodium through consumption of food items with the highest contribution to sodium intake at 4 years of age (mean sodium intake per week)For this study, the dietary exposure to sodium was divided into categories according to the quartiles distribution in our sample.
H. pylori infection statusWhole-cell IgG antibodies against H. pylori were quantified using enzyme-linked immunosorbent assay (ELISA) (EuroImmun, Luebeck, Germany). With regards to the H. pylori infection status of each participant, results were considered negative when the IgG concentrations were below 16RU/ml, borderline when 16–22RU/ml, and positive when infection titers were 22RU/ml or more. For this analysis, participants with borderline IgG titers were classified as infected.
Statistical analysisSample characteristics were described through proportions, compared using chi-square test or Fisher's exact test, when applicable, and median values, compared using the Kruskal–Wallis test. To quantify the association between dietary exposure to sodium and H. pylori infection, odds ratio (OR) and corresponding 95% confidence intervals (CI) were computed using logistic regression. Multivariate models were used to take into account the possible confounding effect of age and sex of the child, number of siblings, caregiver since birth, and mother's age, country of birth, education and H. pylori infection status (in those living with their mothers), and monthly family income. These variables were chosen either because they showed a significant effect in the univariate model or because they have been described as being associated with our exposure and/or outcome in the literature. Family features used in the models referred to data collected at birth. For sodium intake, the multivariate model was further adjusted for total energy intake. All analyses were carried out using STATA® (version 11.2, 2009; StataCorp LP, College Station, Texas, USA).
ResultsWe found that 28.2% of children were infected with H. pylori at 4 years of age, with 5% of them presenting a borderline result for the anti-H. pylori IgG antibodies in the ELISA test.
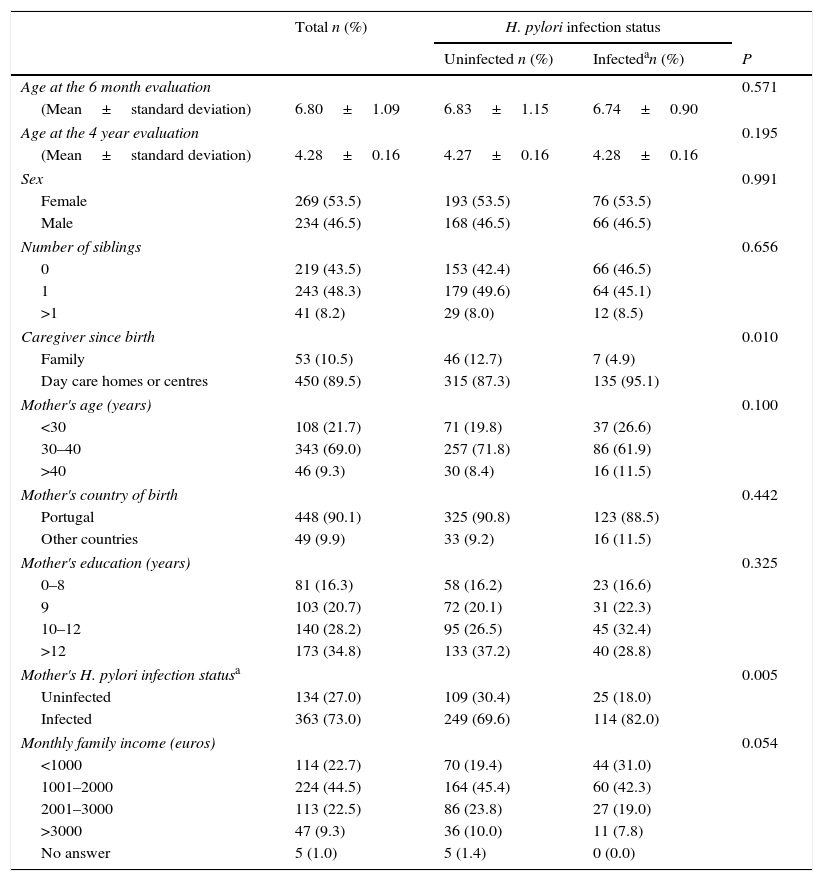
The children participating in this study had a mean age of 6.80 months at the 6 month evaluation and a mean of 4.28 years at the 4 year evaluation, and no differences were observed in age between infected and uninfected children in both evaluations. Most of these children were girls (53.5%), had siblings (56.5%) and had attended day care settings until the 4 year evaluation (89.5%). Regarding their mothers, most of them were aged between 30 and 40 years (69.0%), were born in Portugal (90.1%), had 10 or more years of schooling (63.0%), and were infected with H. pylori (73.0%). Most of the families of these children had a monthly income between 1001 and 2000 Euros (44.5%). The proportion of children that had attended day care settings and were living with their H. pylori-infected mothers was higher among children infected with H. pylori (Table 1).
Children's Helicobacter pylori infection status at 4 years of age, according to socio-demographic characteristics of the children and their families, caregiver since birth and infection status of the mother.
| Total n (%) | H. pylori infection status | |||
|---|---|---|---|---|
| Uninfected n (%) | Infectedan (%) | P | ||
| Age at the 6 month evaluation | 0.571 | |||
| (Mean±standard deviation) | 6.80±1.09 | 6.83±1.15 | 6.74±0.90 | |
| Age at the 4 year evaluation | 0.195 | |||
| (Mean±standard deviation) | 4.28±0.16 | 4.27±0.16 | 4.28±0.16 | |
| Sex | 0.991 | |||
| Female | 269 (53.5) | 193 (53.5) | 76 (53.5) | |
| Male | 234 (46.5) | 168 (46.5) | 66 (46.5) | |
| Number of siblings | 0.656 | |||
| 0 | 219 (43.5) | 153 (42.4) | 66 (46.5) | |
| 1 | 243 (48.3) | 179 (49.6) | 64 (45.1) | |
| >1 | 41 (8.2) | 29 (8.0) | 12 (8.5) | |
| Caregiver since birth | 0.010 | |||
| Family | 53 (10.5) | 46 (12.7) | 7 (4.9) | |
| Day care homes or centres | 450 (89.5) | 315 (87.3) | 135 (95.1) | |
| Mother's age (years) | 0.100 | |||
| <30 | 108 (21.7) | 71 (19.8) | 37 (26.6) | |
| 30–40 | 343 (69.0) | 257 (71.8) | 86 (61.9) | |
| >40 | 46 (9.3) | 30 (8.4) | 16 (11.5) | |
| Mother's country of birth | 0.442 | |||
| Portugal | 448 (90.1) | 325 (90.8) | 123 (88.5) | |
| Other countries | 49 (9.9) | 33 (9.2) | 16 (11.5) | |
| Mother's education (years) | 0.325 | |||
| 0–8 | 81 (16.3) | 58 (16.2) | 23 (16.6) | |
| 9 | 103 (20.7) | 72 (20.1) | 31 (22.3) | |
| 10–12 | 140 (28.2) | 95 (26.5) | 45 (32.4) | |
| >12 | 173 (34.8) | 133 (37.2) | 40 (28.8) | |
| Mother's H. pylori infection statusa | 0.005 | |||
| Uninfected | 134 (27.0) | 109 (30.4) | 25 (18.0) | |
| Infected | 363 (73.0) | 249 (69.6) | 114 (82.0) | |
| Monthly family income (euros) | 0.054 | |||
| <1000 | 114 (22.7) | 70 (19.4) | 44 (31.0) | |
| 1001–2000 | 224 (44.5) | 164 (45.4) | 60 (42.3) | |
| 2001–3000 | 113 (22.5) | 86 (23.8) | 27 (19.0) | |
| >3000 | 47 (9.3) | 36 (10.0) | 11 (7.8) | |
| No answer | 5 (1.0) | 5 (1.4) | 0 (0.0) | |
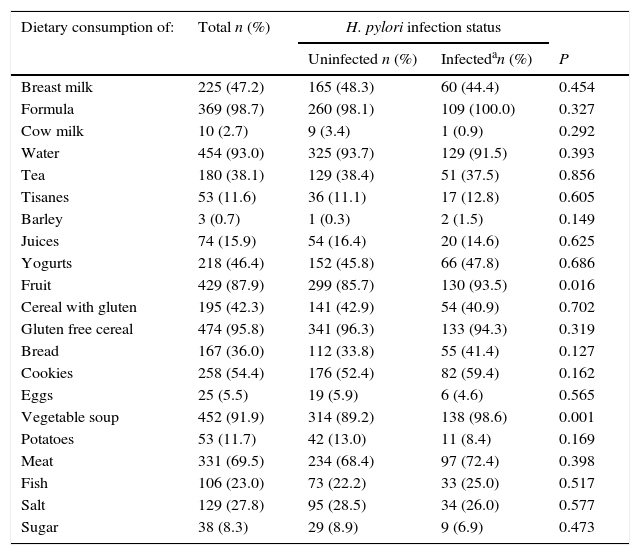
At the 6 month evaluation, less than half of the children were still being breastfed, and in almost all of them, other types of milk were already being consumed. The proportion of children consuming fruit and vegetable soup was higher among the infected (93.5% vs. 85.7%, p=0.016 and 98.6% vs. 89.2%, p=0.001, respectively). With regards to other food items, no statistically significant differences were observed between infected and uninfected. However, 27.8% and 8.3% of the children had already started to consume salt and sugar, respectively, at 6 months of age. Bread was consumed by 36.0% of the children and cookies were part of the diet for 54.4%. Along with vegetable soup, water and gluten free cereals were the food items that most children were consuming at 6 months of age (93.0% and 95.8%, respectively). On the other hand, eggs were the food item less consumed by the children at this age (5.5%) (Table 2).
Children's Helicobacter pylori infection status at 4 years of age, according to dietary habits reported in the 6 month evaluation.
| Dietary consumption of: | Total n (%) | H. pylori infection status | ||
|---|---|---|---|---|
| Uninfected n (%) | Infectedan (%) | P | ||
| Breast milk | 225 (47.2) | 165 (48.3) | 60 (44.4) | 0.454 |
| Formula | 369 (98.7) | 260 (98.1) | 109 (100.0) | 0.327 |
| Cow milk | 10 (2.7) | 9 (3.4) | 1 (0.9) | 0.292 |
| Water | 454 (93.0) | 325 (93.7) | 129 (91.5) | 0.393 |
| Tea | 180 (38.1) | 129 (38.4) | 51 (37.5) | 0.856 |
| Tisanes | 53 (11.6) | 36 (11.1) | 17 (12.8) | 0.605 |
| Barley | 3 (0.7) | 1 (0.3) | 2 (1.5) | 0.149 |
| Juices | 74 (15.9) | 54 (16.4) | 20 (14.6) | 0.625 |
| Yogurts | 218 (46.4) | 152 (45.8) | 66 (47.8) | 0.686 |
| Fruit | 429 (87.9) | 299 (85.7) | 130 (93.5) | 0.016 |
| Cereal with gluten | 195 (42.3) | 141 (42.9) | 54 (40.9) | 0.702 |
| Gluten free cereal | 474 (95.8) | 341 (96.3) | 133 (94.3) | 0.319 |
| Bread | 167 (36.0) | 112 (33.8) | 55 (41.4) | 0.127 |
| Cookies | 258 (54.4) | 176 (52.4) | 82 (59.4) | 0.162 |
| Eggs | 25 (5.5) | 19 (5.9) | 6 (4.6) | 0.565 |
| Vegetable soup | 452 (91.9) | 314 (89.2) | 138 (98.6) | 0.001 |
| Potatoes | 53 (11.7) | 42 (13.0) | 11 (8.4) | 0.169 |
| Meat | 331 (69.5) | 234 (68.4) | 97 (72.4) | 0.398 |
| Fish | 106 (23.0) | 73 (22.2) | 33 (25.0) | 0.517 |
| Salt | 129 (27.8) | 95 (28.5) | 34 (26.0) | 0.577 |
| Sugar | 38 (8.3) | 29 (8.9) | 9 (6.9) | 0.473 |
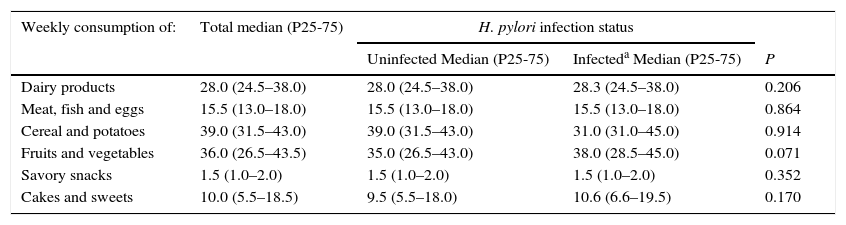
At 4 years of age, the median daily sodium intake was 2364.8mg, and no differences were observed between infected and uninfected children (median daily sodium intake: 2389.9mg vs. 2304.6mg, p=0.559). At this age, “cereals and potatoes” and “fruits and vegetables” were the most consumed food groups on a weekly basis (more than 3 times a day), followed by “dairy products” (2 times a day) and “meat, fish and eggs” (≈once a day). Daily consumption was also observed for cakes and sweets whereas savory snacks were consumed about once a week (Table 3). No statistically significant differences were found between infected and uninfected children when comparing their weekly intake of the food items with the highest contribution to sodium intake (Table 3).
Children's Helicobacter pylori infection status at 4 years of age, according to dietary habits reported in the 4 year evaluation.
| Weekly consumption of: | Total median (P25-75) | H. pylori infection status | ||
|---|---|---|---|---|
| Uninfected Median (P25-75) | Infecteda Median (P25-75) | P | ||
| Dairy products | 28.0 (24.5–38.0) | 28.0 (24.5–38.0) | 28.3 (24.5–38.0) | 0.206 |
| Meat, fish and eggs | 15.5 (13.0–18.0) | 15.5 (13.0–18.0) | 15.5 (13.0–18.0) | 0.864 |
| Cereal and potatoes | 39.0 (31.5–43.0) | 39.0 (31.5–43.0) | 31.0 (31.0–45.0) | 0.914 |
| Fruits and vegetables | 36.0 (26.5–43.5) | 35.0 (26.5–43.0) | 38.0 (28.5–45.0) | 0.071 |
| Savory snacks | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 0.352 |
| Cakes and sweets | 10.0 (5.5–18.5) | 9.5 (5.5–18.0) | 10.6 (6.6–19.5) | 0.170 |
P25-75 – percentiles 25–75;
No statistically significant associations were observed between levels of dietary exposure to sodium and H. pylori infection status, with the exception of the 2nd quarter in the score concerning the consumption of “other food items” in the assessment at 6 months of age (OR=2.41; 95%CI: 1.29–4.50) (Table 4). Therefore, no consistent trend was found between increasing levels of sodium intake and H. pylori infection status in our sample (Table 4).
Children's Helicobacter pylori infection status at 4 years of age, according to dietary exposure to sodium at the 6 month and 4 year evaluations.
| Exposure to sodium through the consumption of: | H. pylori infection status | |||
|---|---|---|---|---|
| Uninfected | Infecteda | |||
| n (%) | n (%) | OR (95%CI)b | ||
| At 6 months | Milks (mean sodium intake-months) | |||
| 1st quarter | 97 (26.9) | 36 (25.4) | 1 | |
| 2nd quarter | 82 (22.7) | 37 (26.1) | 1.29 (0.72–2.30) | |
| 3rd quarter | 95 (26.3) | 32 (22.5) | 0.75 (0.41–1.40) | |
| 4th quarter | 87 (24.1) | 37 (26.1) | 1.14 (0.64–2.08) | |
| Other food items (mean sodium intake-months) | ||||
| 1st quarter | 100 (27.7) | 26 (18.3) | 1 | |
| 2nd quarter | 78 (21.6) | 47 (33.1) | 2.41 (1.29–4.50) | |
| 3rd quarter | 91 (25.2) | 37 (25.4) | 1.61 (0.79–3.26) | |
| 4th quarter | 92 (25.5) | 33 (23.2) | 1.38 (0.69–2.77) | |
| At 4 years | Food items with the highest contribution to sodium intake (mean sodium intake/week) | |||
| 1st quarter | 93 (25.8) | 33 (23.2) | 1 | |
| 2nd quarter | 88 (24.4) | 38 (26.8) | 1.16 (0.65–2.06) | |
| 3rd quarter | 85 (23.6) | 41 (28.9) | 1.25 (0.70–2.24) | |
| 4th quarter | 95 (26.3) | 30 (21.1) | 0.87 (0.47–1.60) | |
| Sodium intake (mg/day) | ||||
| 1st quarter | 60 (25.8) | 19 (23.2) | 1 | |
| 2nd quarter | 52 (22.3) | 27 (32.9) | 1.55 (0.74–3.28) | |
| 3rd quarter | 61 (26.2) | 17 (20.7) | 0.88 (0.40–1.96) | |
| 4th quarter | 60 (25.8) | 19 (23.2) | 1.01 (0.46–2.23) | |
No association between sodium intake and H. pylori infection was found, however a consumption of fruit and vegetable soup at 6 months of age was observed among infected children. This study also shows that nearly 30% of the children were infected with H. pylori at 4 years of age, and the daily sodium intake exceeded the World Health Organization recommendation (<2g/day13) in 26% of the children at this age.
In a previous study using the birth cohort G21, a high prevalence of H. pylori infection had already been reported, showing an increasing risk of infection with the cumulative time of attendance in day care centres or homes since birth,14 in line with a previous meta-analysis that concluded that this association was stronger in high prevalence settings.15 These results are also in accordance with other studies conducted in Portugal, in adolescents16 and in adults.17 However, H. pylori prevalence is higher in the Northern region of the country2 compared with what has been reported in the South,18 where 20% of children aged 0–5 years were infected and prevalence increased with age. The prevalence found here is also higher than in other countries,19 making this setting an ideal target for intervention.
Although 83% of the children in our study were above the Maximum Tolerable Daily Intake for sodium (>1.9g/day20), at 4 years of age, the mean daily sodium intake was slightly lower than the value reported for the US population in this age group (2375mg/day vs. 2864mg/day), both evaluated by a food item questionnaire.20 In France, in 1998–1999, sodium intake was also above the recommended in both children and adults, with an excessive intake from early life and increasing with age.21 This reflects the global increase in sodium intake observed between 1990 and 2010, leading to excessive sodium intakes in almost all countries.22
Several studies have used data on urinary excretion, which is considered to be the gold-standard to evaluate sodium intake, especially when through a 24-h urine collection. Food frequency questionnaires may under or overestimate actual dietary sodium intake.23,24 In Germany, in 2003–2009, children and adolescents presented a urinary sodium excretion of 1.4g/day and 3.2g/day, respectively.25 In Portugal, in 2010, an average sodium intake of 3120mg/day based on 24-h urinary sodium excretion was reported for children aged 10–12 years.26 In 2014, the mean sodium excretion was found to be higher in boys compared with girls, among children aged 8–10 years with a daily mean of 2657mg in both sexes.27 A similar result was found in a subsample of the G21 cohort, evaluated at 8–9 years, with an average estimated sodium intake among children enrolled in the study of 2600mg/day 28. Although the measurement of sodium content in a 24h-urine sample was not available for our study, we used several methods for sodium intake estimation, such as through the consumption of different types of milk, in which data on the sodium content of every milk registered by the parents was retrieved, and taking into account the sodium content of the food items that had been consumed by the children at the 6 month evaluation. In both cases, we computed the time interval of exposure. In addition, we used the list of food items with the highest contribution to sodium intake at 4 years of age,12 and estimated the weekly sodium intake. The daily mean sodium intake was retrieved from the 3-day food dairies, as reported by the parents. Nevertheless, the methods we used probably underestimated the true dietary sodium intake in these children, resulting in weaker associations than if sodium excretion was available at 4 years.
Since there is evidence that when H. pylori is in an environment with high sodium content, it increases its resistance by expressing CagA,29 it would have been important to understand if this effect is present in our sample. However, no information was available concerning the various subtypes of bacteria, and it was not possible to evaluate the resistance of these strains to high sodium concentrations.
Although no statistically significant association between sodium intake and H. pylori infection was observed, we found a higher proportion of infected children among those consuming vegetable soup and fruit according to reports at 6 months of age. This result can be justified by the fact that in Portugal, vegetable soup is a food item that highly contributes to sodium intake.30,31 Also, in France, in both children and adults, it was found that bread, vegetable soup, cooked pork, convenience foods, sweets and sugary products were mainly responsible for high sodium intake.21 On the other hand, the ease of handling fruit may lead to problems of hygiene and food safety, contributing to the spread of infection. The way that fruit is washed before consumption, as well as possible contamination of water, can be factors that increase the risk of H. pylori infection by fruit consumption.32 In fact, a previous study has shown that H. pylori transmission can occur by several routes, namely toys at the day care centre.33
In this study, 73.4% of the mothers were infected with H. pylori, which, according to data from the general population, is lower than expected for this age group with these years of schooling.17 Since most mothers of participating children had more than 10 years of schooling and were aged above 30 years, we can consider the possibility of a selection bias, which may have contributed to the lack of a statistically significant association between sodium intake and infection by H. pylori in our results. For instance, in Portuguese women, the consumption of bread, a food item that highly contributes to sodium intake, tended to decrease with increasing education.34 In fact, the mothers included in this analysis rated their dietary habits as being equally healthy during work days and the weekend, while the excluded mothers reported that their dietary habits during the weekend tended to be less healthy. This can lead us to assume that our results are underestimated, since it is likely that these mothers are more aware of the dietary needs of their children, which may have also resulted in an underestimation of the prevalence the infection.
Although outside the scope of this work, the confirmation of an excessive sodium intake in these children highlights the need for the promotion of strategies that facilitate the adoption of different preventive interventions, including health education, with the participation of the whole family. Pregnancy should be seen as an opportunity for prevention, with participation in nutritional education sessions and workshops, not only for the mother but also the father and close family members such as grandparents, may be a viable strategy for reducing sodium intake in the early stages of life. This type of intervention, which has already been established for breastfeeding,35 leads to a greater understanding, responsibility and change of attitude by the stakeholders and thus improving the health of this and future generations. Nutrition education for children in schools is also a good way to convey information to the families; however, it is necessary that they also feel involved in this type of intervention so that the sharing of information that exists in schools is also experienced at home. It is important that there is a synergy between the educational community, the family, the food industry, health professionals and politicians,36,37 so that it is possible to implement continuous actions on nutrition education and in this particular case to reduce sodium intake in populations.
Although there is a high prevalence of H. pylori infection in our sample, there were no statistically significant differences in the results obtained, which does not allow us to infer that sodium is one of the causes for H. pylori infection. To the best of our knowledge, the present epidemiologic study is the first to test the influence of sodium intake in H. pylori infection in children, as until now this hypothesis had only been tested in animal studies. If the relation between sodium intake and H. pylori infection status had been confirmed, the identification of this cause-effect would allow for defining strategies to prevent infection from a very early stage in life. Thus, further studies are needed in this area, using different methods for assessing sodium intake and an analysis of specific H. pylori subtypes in this age group.
FundingG21 was funded by Programa Operacional de Saúde – Saúde XXI, Quadro Comunitário de Apoio III and Administração Regional de Saúde Norte (Regional Department of Ministry of Health). It has support from the Portuguese Foundation for Science and Technology, trough Fundo Europeu de Desenvolvimento Regional (FEDER) by the Programa Operacional Factores de Competitividade (POFC) – COMPETE (FCOMP-01-0124-FEDER-011000) and through national funds (PTDC/SAU-ESA/103958/2008), and from the Calouste Gulbenkian Foundation. The work at the Epidemiology Research Unit – Institute of Public Health, University of Porto (UID/DTP/047507/2013) and the individual grants attributed to BP (SFRH/BPD/75918/2011) and ARC (SFRH/BD/102181/2014) were supported by Portuguese Foundation for Science and Technology.
Conflicts of interestThe authors declare no conflicts of interest.
The authors gratefully acknowledge the families enrolled in Generation XXI for their kindness, all members of the research team for their enthusiasm and perseverance and the participating hospitals and their staff for their help and support.