The aim of this study was to evaluate the effect of stopping the agonist with the first follow up visit when the initial response was poor in cases undergoing intracytoplasmic sperm injection, comparing this to the conventional continuation of the agonist.
MethodologyA prospective single blinded study was conducted on 50 infertile cases who were planned to have intracytoplasmic sperm injection by long protocol in infertility unit of El-Shatby Maternity University Hospital in the period between May 2011 and January 2013 and these patients had poor response on day 6 of stimulation (serum estradiol (E2) less than 100pg/ml and 5 or less follicles developed). They were randomly allocated by closed envelope method into two groups, (I) 30 patients to whom we stopped the agonist and continued stimulation and (II) 20 patients to whom we continued the agonist together with stimulation.
ResultsGroup II had statistically higher days of stimulation than group I (p=0.009). The number of metaphase II oocytes did not show statistical significant difference between the two groups (p=0.418). The mean of number embryos replaced were statistically higher in group II than group I (p=0.025). Pregnancy rate showed no statistical significant differences between the two studied groups (p=0.466).
ConclusionsThere was no difference between stopping the agonist or continuing it when the initial response was poor on first follow up visit in cases undergoing intracytoplasmic sperm injection.
RecommendationsAlternative measures should be looked for, to improve results of intracytoplasmic sperm injection when initial response is poor.
O objetivo desse estudo foi avaliar o efeito da interrupção do agonista por ocasião da primeira consulta de seguimento, quando a resposta foi insatisfatória em casos tratados com injeção intracitoplasmática de espermatozoide (IICE), e comparar essa medida com a continuação convencional do agonista.
MetodologiaUm estudo prospectivo monocego foi realizado em 50 casos de infertilidade, com planejamento de IICE por protocolo longo na Unidade de Infertilidade da Maternidade do Hospital Universitário El Shatby no período entre maio de 2011 e janeiro de 2013; essas pacientes tiveram resposta insatisfatória no Dia 6da estimulação (estradiol sérico [E2]<100 pg/ml e desenvolvimento de 5 ou menos folículos). As pacientes foram randomicamente designadas pelo método do envelope fechado para a formação de dois grupos: Grupo I, 30 pacientes que tiveram interrompida a medicação com o agonista e continuaram com a estimulação, e Grupo II, 20 pacientes que continuaram com o agonista juntamente com a estimulação.
ResultadosO Grupo II apresentou um número estatisticamente maior de dias de estimulação versus Grupo I (p=0,009). O número de ovócitos em metáfase II não exibiu diferença estatisticamente significativa entre os dois grupos (p=0,418). O número médio de embriões recuperados foi estatisticamente maior no Grupo II versus Grupo I (p=0,025). O percentual de gestações não revelou diferenças estatisticamente significativas entre os dois grupos estudados (p=0,466).
ConclusõesEm casos tratados com IICE, não foi observada diferença entre a interrupção do agonista ou sua continuação, quando a resposta inicial foi insatisfatória na primeira consulta de seguimento.
RecomendaçõesÉ preciso se pensar em medidas alternativas para melhorar os resultados da IICE, nos casos em que a resposta inicial não for satisfatória.
Low ovarian response to stimulation protocol in intracytoplasmic sperm injection (ICSI) is frustrating to physicians and patients especially when the initial evaluation seems to be fair. Regarding this emerging problem, different measures usually taken trying to rescue the cycle and avoid cancelation. It was estimated that about 9–18% of cycles result in this low response with small number of follicles and low estradiol level.1
The term “poor responder” has been used to determine women who require large doses of stimulation medications and who make less than an optimal number of eggs. The definition of low responders differs from center to other, but the most used one is that less than four dominant follicles on day of human chorionic gonadotropin (hCG) administration.2
The occurrence of low response to ovarian stimulation is usually suspected in old age, although it could occur at any age. Ovarian reserve is the main factor affecting response to stimulation, it could be assessed by many parameters as basal follicle stimulating hormone (FSH), antral follicle count (AFC) and anti-mullarian hormone (AMH). These parameters could be normal, yet poor response happen. A lot of explanations trying to answer the question of: why low ovarian reserve in young patients.3
The optimal protocol to deal with poor responders is controversial, the same as the definition. There are many trials, including the use of higher doses of gonadotropins, different doses of gonadotropin releasing hormone (GnRH) agonists, adding estrogen or growth hormone, using combined oral contraceptive pills or the use of natural cycle.1,2
The long GnRH agonist protocol is the most widely used and is the preferred for women planned to have ICSI. In poor responders, release of this suppressive effect of GnRH may change the response of the ovary by opening the ovarian receptors. The concept of: GnRH agonist stop protocols developed depending on this theory.4,5
These protocols are characterized by the use of somewhat low doses of GnRH agonists from the mid-luteal phase of the cycle till the time of menses or soon later, this usually occurs in combination with high doses of gonadotropins. Although GnRH agonist is stopped, it was found that the occurrence of unprogrammed luteinizing hormone (LH) surge is still low.4,5
Materials and methodsThe aim of the study was to compare the results of ICSI in cases of poor response on day 6 stimulation of long protocol between patients who stopped the agonist and those who continued it.
To reach this aim, this randomized controlled single blinded study was conducted on 50 infertile cases who were planned to have ICSI by long agonist protocol in infertility unit of El-Shatby Maternity University Hospital in the period between May 2011 and January 2013.
These patients had poor response on D6 of stimulation (serum estradiol (E2) less than 100pg/ml and 5 or less follicles developed) and were randomly allocated by closed envelope method into two groups: Group I: 30 patients to whom we stopped the agonist and continued stimulation. Group II: 20 patients to whom we continued the agonist together with stimulation.
All patients were assessed by basal FSH and AFC and they were within normal range beside the routine assessment of infertile couple (transvaginal ultrasound, HSG and semen analysis) and they were candidates for ICSI.
The protocol used was the long agonist protocol in which: (a) pituitary desensitization was performed by the use of gonadotropin releasing hormone (GnRh) agonist (decapeptyl 0.1mg, Ferring) in a daily subcutaneous dose started on cycle day 21. On second day of menses, pituitary downregulation was assured and the dose was reduced to 0.05mg and continued until day 6 of stimulation. (b) Ovarian stimulation with urinary human menopausal gonadotropin (u-hMG) (Merional_, IBSA) 75IU and urinary follicle stimulating hormone (u-FSH) (Fostimon_, IBSA) 75IU. The standard initial dose was 300IU started from the third day of the cycle. (c) First assessment of ovarian response to stimulation is done on D6 (after 5 days of stimulation), those who have E2 less than 100pg/ml and 5 or less follicles developed were enrolled in the study and were randomly allocated into two groups: Group I: 30 patients to whom we stopped the agonist and continued stimulation. Group II: 20 patients to whom we continued the agonist together with stimulation. And for all patients we increased the dose of stimulation to 450IU. (d) Follow up of ovulation was done every other day by estimating E2 and transvaginal ultrasound and the dose of stimulation was adjusted till the criteria for administration of hCG was reached. (e) Ovulation was induced when there were 2 or more follicles greater than 18mm in diameter by administration of hCG (Choriomon_, IBSA) 10,000IU subcutaneous (s.c.) or intramuscular (i.m.) injection. Ovum pick up was done 35h after hCG administration guided by transvaginal ultrasound. Embryo transfer was done on D3 under ultrasound guidance, and was considered statistically significant. All analyses were performed using SPSS for Windows, version 18.0.
ResultsData collectionOrientation and official approval from relevant authorities: collaborative agreement.
In order to enable the researcher to conduct the study, the necessary permissions to conduct the study were obtained. Ethical consideration: The confidentiality of collected data was stressed and a written informal consent was taken from all women.
Data analysisThe chi-square (χ2) test was used to analyze categorical variables which were expressed as percentage values. Continuous variables were reported as mean value and standard deviation (SD) and analyzed using the t-test. The continuous variables were also reported as median and analyzed using Mann–Whitney test. A p-value was found to be <0.05.
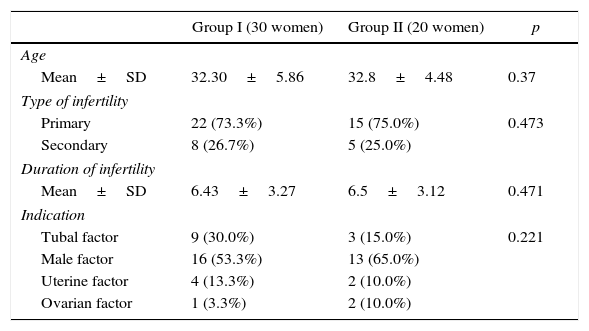
Demographic dataDemographic data and clinical data of the two studied groups were presented in Table 1. It demonstrated that: the mean age was 32.30±5.86 and 321.8±4.48 for groups I and II, respectively; there were no statistical significant differences between the two studied groups regarding age (p=0.37). Primary infertility was 22 (73.3%) and 15 (75.0%), and secondary infertility was 8 (26.7%) and 5 (25.0%) for groups I and II, respectively; there were no statistical significant differences between the two studied groups regarding type of infertility (p=0.473). The mean of duration of infertility was 6.43±3.27 and 6.5±3.12 for groups I and II, respectively; there were no statistical significant differences between the two studied groups (p=0.471).
Demographic and clinical data of the two studied groups.
| Group I (30 women) | Group II (20 women) | p | |
|---|---|---|---|
| Age | |||
| Mean±SD | 32.30±5.86 | 32.8±4.48 | 0.37 |
| Type of infertility | |||
| Primary | 22 (73.3%) | 15 (75.0%) | 0.473 |
| Secondary | 8 (26.7%) | 5 (25.0%) | |
| Duration of infertility | |||
| Mean±SD | 6.43±3.27 | 6.5±3.12 | 0.471 |
| Indication | |||
| Tubal factor | 9 (30.0%) | 3 (15.0%) | 0.221 |
| Male factor | 16 (53.3%) | 13 (65.0%) | |
| Uterine factor | 4 (13.3%) | 2 (10.0%) | |
| Ovarian factor | 1 (3.3%) | 2 (10.0%) | |
SD, standard deviation.
Laboratory and maternal findings in the two studied groups were presented in Table 2. It illustrated that: the mean of D3 FSH was 7.56±1.66 and 7.04±1.32 for groups I and II respectively; there were no statistical significant differences (p=0.119).
Laboratory and maternal findings in the two studied groups.
| Group I (30 women) | Group II (20 women) | p | |
|---|---|---|---|
| D3 FSH | 7.56±1.66 | 7.04±1.32 | 0.119 |
| AFC | 6.93±2.16 | 9.30±1.78 | 0.0001 |
| D6 E2 | 70.97±14.67 | 67.15±20.70 | 0.224 |
| D6 response | 4.20±0.71 | 4.00±0.92 | 0.196 |
| Days of stimulation | 11.73±0.98 | 12.55±1.39 | 0.009a |
| Final E2 | 708.30±195.37 | 863.60±178.17 | 0.003a |
| Number of oocytes | 4.77±1.55 | 5.00±1.59 | 0.304 |
| Metaphase II oocytes | 4.00±1.60 | 4.10±1.77 | 0.418 |
| Number of embryo(s) | 2.00±1.44 | 2.65±1.39 | 0.059 |
| Number of embryo(s) transferred | 1.87±1.33 | 2.65±1.39 | 0.025a |
FSH, follicle stimulating hormone; AFC, antral follicle count.
The mean of AFC was 6.93±2.16 and 9.30±1.78 for groups I and II, respectively; group II has values statistically higher than group I (p=0.0001). The mean of D6 E2 was 70.97±14.67 and 67.15±20.70 for groups I and II, respectively; there were no statistical significant differences (p=0.224). The mean of D6 response was 4.20±0.71 and 4.00±0.92 for groups I and II, respectively; there were no statistical significant differences (p=0.196). The mean of days of stimulation was 11.73±0.98 and 12.55±1.39 for groups I and II, respectively; group II has values statistically higher than group I (p=0.009). The mean of final E2 was 708.30±195.37 and 863.60±178.17 for groups I and II, respectively; group II has values statistically higher than group I (p=0.003). The mean of number of oocytes was 4.77±1.55 and 5.00±1.59 for groups I and II, respectively; there were no statistical significant differences (p=0.304). The mean of metaphase II was 4.0±1.60 and 4.10±1.77 for groups I and II, respectively; there were no statistical significant differences (p=0.418). The mean of number of embryo was 2.0±1.44 and 2.65±1.39 for groups I and II, respectively; there were no statistical significant differences (p=0.059). The mean of number of embryo replaced was 1.87±1.33 and 2.65±1.39 for groups I and II, respectively; group II has values statistically higher than group I (p=0.025).
Pregnancy rateTable 3 shows pregnancy rate in the two studied groups. It demonstrated that pregnancy rate was 23.3% and 20.0% for groups I and II, respectively; there were no statistical significant differences between the two studied groups (p=0.466).
DiscussionThe results of our study showed that cessation of agonist on D6 of stimulation when there is poor response does not differ from continuation of the agonist as regard ICSI outcomes.
The value of agonist is to prevent premature LH surge and thus prevent cycle cancelation, however, this may need higher doses of gonadotropins.6
Although many studies used early discontinuation of the agonist, the incidence of premature LH surge was very low; in spite of this, the results are still contradictory.
The possible mechanism of action of agonist-stop protocol is the reduced effect of the GnRH agonists on their ovarian receptors and this leads to reduced ovarian suppression and so increase ovarian response.4,5
Another possible mechanism is that GnRH agonists decrease follicular blood flow as proved by some studies and stop of the agonist restores follicular blood flow with no effect on pituitary suppression and this helps to recruit more follicles and gives better ICSI outcomes.7–10
There are a lot of studies published about early cessation of agonist and the results differ, only two of these studies were prospective randomized controlled trials and showed no statistically significant difference in pregnancy rates. Other studies were prospective trials with historical controls or retrospective demonstrated improved pregnancy outcome.
One prospective randomized, controlled trial involving 78 cycles, a “stop agonist” regimen was compared with a standard long luteal protocol. GnRH agonist (buserelin 1mg/day intranasally (i.n.) or triptorelin 0.1mg/day, s.c.) was started day 21 of the preceding cycle and stopped with pituitary suppression and start of stimulation. Ovarian stimulation with a dose of 225–375IU/day hMG or purified FSH. The results showed no improvement because the mean number of the retrieved oocytes did not change and the pregnancy rate did not show significant difference.11
Another prospective, randomized, controlled trial compared “stop” versus “non-stop” protocol of GnRH agonist, together with high doses of gonadotropins. Leuprolide (1mg, s.c.) was used from day 21 of the cycle and stopped on the day of menses, followed by stimulation of ovulation by a dose of 375–450IU hMG and/or purified FSH daily. The results of this study showed a significantly higher number of retrieved oocytes (8.7 versus 5.3 per cycle, p=0.027) and higher number of mature oocytes, but there were similar fertilization and cleavage rates as well as similar numbers of embryos transferred. There was no significant difference in either cancelation rate (5.7% versus 2.8%) or pregnancy rate (18.7% versus 14.3%).12
The other studies did not show the same results, they were prospective trials but used historical controls. One of them studied 224 cycles, in these cycles a low-dose mid-luteal GnRH agonist (leuprolide 0.5mg, s.c.) was given and stopped with the onset of menses and this was named by the authors as the “stop-Lupron protocol”. Ovulation induction was done by a dose of 450–600IU of purified FSH or hMG (i.m.) daily. The dose of gonadotropins was decreased 2 days prior to hCG administration, The results of this study showed low cancelation rate (12.5%), high number of retrieved oocytes (11.1), and higher clinical pregnancy rate per transfer (32%).13
A non-randomized prospective study used 52 cycles and administered 0.5mg leuprolide s.c. from day 21 to the next cycle day 2 and stopped leuprolide. Ovulation was induced with a dose of 300–450IU/day hMG and purified FSH. The results of this study showed that there were a good response to stimulation (mean 7.5 oocytes per cycle) as well as better pregnancy rates (20.5% per embryo transfer).14
Another prospective study with historical controls used 82 cycles and administered 0.5mg leuprolide s.c. from day 21 to the next cycle day 2 and stopped leuprolide. Ovulation was induced with a dose of 450–600IU/day hMG and purified FSH. The results of this study showed that there were high pregnancy rates (33.3%) and low cancelation rates (31.6%).15
Another prospective study with historical controls which involved 36 poor responders and used nafarelin (0.6mg/day) started in the mid-luteal phase and stooped on day 5 of ovarian stimulation. This protocol stopped the agonist late and this is similar to our protocol but this was planned as these patients were diagnosed as poor responders from the start. Ovulation was induced with a dose of 300IU/day hMG. The results of this study showed increase in the number of retrieved oocytes by 28%, decrease in cancelation rates (to 8.3%) and increase pregnancy rates (to 19.4%).16
In another prospective study with historical controls, and using also nafarelin (0.6mg/day) started in the mid-luteal phase but discontinued on day 1 of the next cycle and included 39 poor responders. Ovulation was induced with a dose of 300IU/day hMG. The results of this study showed increase in the number of retrieved oocytes and the pregnancy rates (10.7% versus 2.8%).17
ConclusionsThere was no difference between stopping the agonist or continuing it when the initial response was poor on first follow up visit in cases undergoing ICSI.
RecommendationsLarger multicentric study is needed to evaluate this unplanned agonist stop protocol.
Conflicts of interestThe authors declare no conflict of interest.