Shigella flexneri is divided into 13 serotypes based on the combination of antigenic determinants present in the O-antigen. A new O-antigen modification with phosphoethanolamine has been identified. The presence of this antigenic determinant (called E1037) is recognized by monoclonal antibody MASF IV-1. Given the increasing incidence of these new variants and the difficulty in supplying the monoclonal antibody to our country, we produced a polyclonal antiserum (AA479) through immunization with a S. flexneri Xv strain. The antiserum specificity was assessed by slide agglutination against isolates from clinical cases and a culture collection representing all Shigella serotypes. The results obtained demonstrated a 100% correlation between AA479 absorbed antiserum and monoclonal antibody MASF IV-1. The availability of AA479 antiserum in every public hospital in Argentina will allow us to identify atypical S. flexneri isolates in order to strengthen Shigella surveillance in our country and to compare with global epidemiological data.
Shigella flexneri se divide en al menos 13 serotipos sobre la base de la combinación de determinantes antigénicos presentes en el antígeno O. Se identificó una nueva modificación del antígeno O con fosfoetanolamina. La presencia de este determinante antigénico (denominado E1037) es reconocida por el anticuerpo monoclonal MASF IV-1. Teniendo en cuenta la incidencia creciente de estas nuevas variantes y la dificultad en la provisión del anticuerpo monoclonal para nuestro país, se elaboró un antisuero de tipo policlonal (AA479) mediante la inmunización con un cultivo de S. flexneri Xv. La especificidad del antisuero se evaluó por aglutinación en lámina con aislamientos clínicos y cultivos de colección, con lo que quedaron representados todos los serotipos de Shigella. Los resultados obtenidos demostraron una correlación del 100% entre el antisuero AA479 absorbido y el anticuerpo monoclonal MASF IV-1. La disponibilidad del antisuero AA479 en todos los hospitales públicos de Argentina permitirá identificar los aislamientos atípicos de S. flexneri; de esta forma se podrá fortalecer la vigilancia de Shigella en nuestro país y comparar con los datos epidemiológicos a nivel global.
Shigella infections are a major health problem worldwide, mainly in developing countries. Approximately 160 million shigellosis cases have been estimated per year around the globe by the World Health Organization with the majority involving children under five years of age8,11.
Shigella flexneri is the prevalent species in developing countries, particularly in Argentina where 70% of the Shigella infections are caused by S. flexneri and about 25% by S. sonnei7. In Argentina, Shigella spp. has been identified by the Laboratory Surveillance System (Ministry of Health), as one of the main causative bacterial agents of diarrhea2.
In order to establish the diversity of the circulating serotypes and their distribution in time and space, it is necessary to determine the antigenic characteristics of the Shigella strains in different regions. For this reason, in Argentina, epidemiological surveillance is performed within the context of the National Network for Diarrhea and Food-borne Pathogens (Diarrhea Network) through serotyping with the antisera produced by the Servicio Antígenos y Antisueros from the Instituto Nacional de Producción de Biológicos (INPB) – ANLIS “Dr. Carlos G. Malbrán”.
The genus Shigella comprises four species: S. dysenteriae, S. flexneri, S. boydii and S. sonnei. Except for S. sonnei, each species contains multiple serotypes based on the structure of the O-antigen. S. dysenteriae and S. boydii have been subdivided into 15 and 20 serotypes, respectively. In the serotyping scheme currently in use, S. flexneri is divided into 13 serotypes based on the combination of the type-specific antigen (I, II, III, IV, V, VI) and the presence of one or more group antigens (3,4–6–7,8)3,9. All S. flexneri serotypes (except S. flexneri 6) share the backbone of the basic O-antigen repeat unit, which is a tetrasaccharide consisting of a single N-acetyl glucosamine and three rhamnose residues. This basic structure is referred to as serotype Y and the addition of glucosyl and/or O-acetyl groups to different sugars in the tetrasaccharide unit forms the basis of serotype conversion. The genes involved in the biosynthesis of the tetrasaccharide are located in the chromosome and the O-antigen modification genes known to date are encoded by temperate bacteriophages or prophages which are integrated into the conserved sites of the host S. flexneri genome1.
In recent years, the emergence of atypical S. flexneri has been reported, such as the provisionally called S. flexneri Xv, S. flexneri Yv and S. flexneri 4av6,10–12,15. All these strains showed agglutination with monoclonal antibody MASF IV-1, which binds to the antigenic determinant E1037 not covered in the S. flexneri serotyping scheme currently in use. The MASF IV-1 positive phenotype involves the presence of a phosphoethanolamine (PEtN) group attached to at least one rhamnose of the tetrasaccharide, due to the activity of the PEtN–transferase encoded in the plasmidic gene lpt-O11.
Considering that atypical S. flexneri (E1037 positive) isolates have been detected in our country since 2009 and in view of the difficulty in supplying the antibody, we have produced a polyclonal antiserum (AA479) to allow the identification of such bacterial strains and to strengthen Shigella surveillance in Argentina.
A randomly selected culture of S. flexneri Xv (#479/09 culture) was used for the production of rabbit polyclonal antiserum AA479 (anti-PEtN antiserum). The purity of the bacteria, the biochemical identification and the morphology of the colony (smoothness) were tested. In order to remove thermolabile surface antigens, the immunizing suspension was prepared by heat denatured whole cells with a concentration of approximately 5×109bacteria/ml.
New Zealand White rabbits, 8 weeks old (approximately 2.5kg), were immunized with a first intramuscular 0.5ml dose and then a series of intravenous inoculation of 0.5, 1.0, 2.0, and two doses of 4.0ml administered at 3–4 day interval. The rabbits were exsanguinated by cardiac puncture 7–10 days after the last injection. Then, the serum was stored at 4°C with 10% glycerin phenolized saline solution as a preservative.
Thirty-four S. flexneri strains (32 S. flexneri Xv, 1 S. flexneri 4av and 1 nontypeable S. flexneri), which showed agglutination with monoclonal antibody MASF IV-1, were used to control the AA479 antiserum. All these studied strains were received (over a one year span), between 2009 and 2010, by the National Reference Laboratory (Servicio Enterobacterias from the Instituto Nacional de Enfermedades Infecciosas (INEI) – ANLIS “Dr. Carlos G. Malbrán”) from human stool samples. Sixty-seven Shigella strains from the Antigens and Antisera Service Collection, including all currently recognized serotypes of the four species (15 S. dysenteriae, 19 S. boydii, 2 S. sonnei and 31 S. flexneri) and S. flexneri 7a5,14 were also used for the antiserum control. All cultures were biochemically characterized as Shigella spp. in accordance with the procedures previously described4 and kept at −70°C in tryptic soy broth with glycerol. Serotyping was performed by a slide agglutination assay using specific antisera for all types and group-factor antigens (INPB) and monoclonal antibody against S. flexneri (MASF IV-1; Reagensia AB, Sweden).
Cell suspensions were employed for the titration of AA479 antiserum. Bacterial suspensions were prepared directly from cultures grown at 37°C for 18h with 1.0ml of 0.5% formalinized physiological saline solution. Twenty microliters (20μl) of the bacterial suspension and 20μl of serial dilutions of antiserum were placed on a glass slide and mixed with the microbiological loop or stick. The slide was tilted gently back and forth for 1min and the appearance or non-appearance of agglutination was observed with an indirect intense light. The titer was defined as the inverse of the highest dilution giving a positive result 3+ (large and small clumps against a slightly cloudy background) or 4+ (large clumps against a clear background).
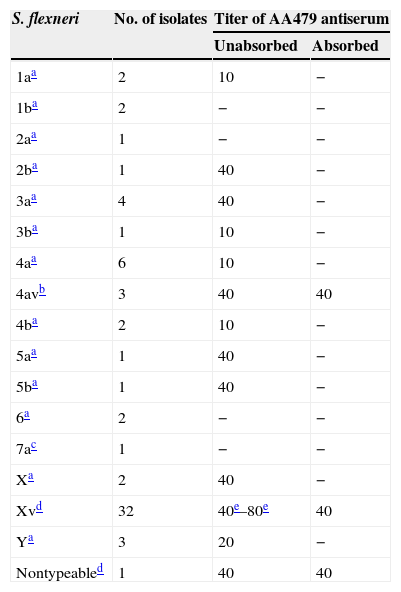
The unabsorbed antiserum showed a strong reaction (titer 40–80) with the collection cultures of S. flexneri 2b, 3a, 4av, 5a, 5b and X serotypes, as well as clinical isolates of S. flexneri 4av, Xv and nontypeable serotypes (Table 1).
Agglutination reactions of AA479 antiserum.
| S. flexneri | No. of isolates | Titer of AA479 antiserum | |
|---|---|---|---|
| Unabsorbed | Absorbed | ||
| 1aa | 2 | 10 | − |
| 1ba | 2 | − | − |
| 2aa | 1 | − | − |
| 2ba | 1 | 40 | − |
| 3aa | 4 | 40 | − |
| 3ba | 1 | 10 | − |
| 4aa | 6 | 10 | − |
| 4avb | 3 | 40 | 40 |
| 4ba | 2 | 10 | − |
| 5aa | 1 | 40 | − |
| 5ba | 1 | 40 | − |
| 6a | 2 | − | − |
| 7ac | 1 | − | − |
| Xa | 2 | 40 | − |
| Xvd | 32 | 40e–80e | 40 |
| Ya | 3 | 20 | − |
| Nontypeabled | 1 | 40 | 40 |
The agglutination absence with the 1/10 diluted antiserum is considered negative (−).
The cultures corresponding to the species S. boydii, S. dysenteriae and S. sonnei were negative in the 1/5 dilution of AA479 antiserum without absorption.
One of the isolates is from a clinical source, the other two belong to the Antigens and Antisera Collection.
S. flexneri 7a culture was kindly provided by Dr. K. Talukder (International Centre for Diarrhoeal Disease Research, Bangladesh).
AA479 antiserum was subjected to a purification process (absorption process) for the removal of heterologous agglutinins in accordance with a previously described procedure4. An overnight growth culture of S. flexneri X at 37°C in tryptic soy agar was used as an absorbent in a ratio of 1 plate (150mm diameter) per 4ml of antiserum. The mixture of cells and serum was incubated for 2h at 50°C in a water bath; then, the absorbed antiserum was removed by centrifugation at 12,000rpm for 30min.
The absorbed antiserum was specific for atypical S. flexneri cultures, including those belonging to 4av, Xv and nontypeable serotypes (Table 1).
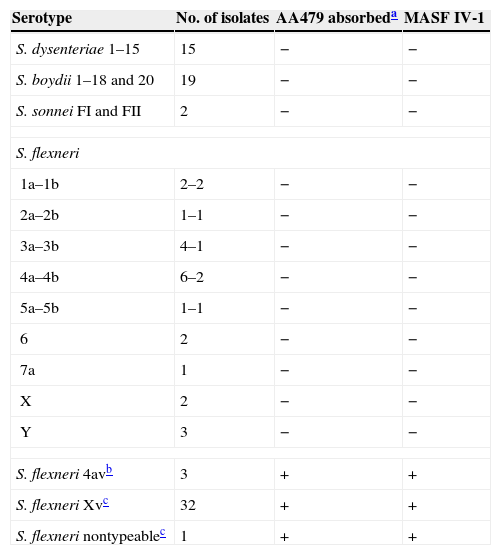
With regard to the correlation between AA479 and MASF IV-1, a 100% concordance between both reagent results was observed (Table 2).
Comparison of the results obtained with AA479 antiserum and monoclonal antibody MASF IV-1.
| Serotype | No. of isolates | AA479 absorbeda | MASF IV-1 |
|---|---|---|---|
| S. dysenteriae 1–15 | 15 | − | − |
| S. boydii 1–18 and 20 | 19 | − | − |
| S. sonnei FI and FII | 2 | − | − |
| S. flexneri | |||
| 1a–1b | 2–2 | − | − |
| 2a–2b | 1–1 | − | − |
| 3a–3b | 4–1 | − | − |
| 4a–4b | 6–2 | − | − |
| 5a–5b | 1–1 | − | − |
| 6 | 2 | − | − |
| 7a | 1 | − | − |
| X | 2 | − | − |
| Y | 3 | − | − |
| S. flexneri 4avb | 3 | + | + |
| S. flexneri Xvc | 32 | + | + |
| S. flexneri nontypeablec | 1 | + | + |
Absorbed AA479 antiserum was used at a dilution of 1/40, according to the titer expressed in Table 1.
Having considered that the emergence of new S. flexneri serotypes is not unusual10,12,13,15, that rapid dissemination of these variants may occur and that multiresistant S. flexneri Xv strains have been detected in China15, it is necessary to keep the epidemiological surveillance of the serotypes of this species updated. Likewise, the information about the distribution of the different serotypes remains important to the proposal of the World Health Organization regarding a vaccine development to decrease the world morbidity caused by Shigella as well as having the knowledge that acquired immunity against this organism infection is serotype-specific8.
AA479 antiserum production and its immediate distribution to the public hospitals belonging to the Diarrhea Network enabled to detect in 2009, the appearance and dissemination of S. flexneri Xv (Viñas, van der Ploeg, et al., unpublished data). This emerging serotype represented 30% of the S. flexneri isolates analyzed in 2010 and became the second serotype in importance after S. flexneri 2, the historically predominant serotype.
S. flexneri unabsorbed antisera are characterized by having cross-reactivity with all of S. flexneri serotype cultures and by the titer decrease of at least one dilution after the absorption process. Nevertheless, AA479 unabsorbed antiserum showed cross-reactivity with only some of the S. flexneri serotypes and its titer did not decrease after absorption. These results may be due to the structural difference of the PEtN group regarding the other O-antigen determinants, mostly glucosyl and rhamnose residues.
The concordance between the results obtained with the AA479 polyclonal antiserum and monoclonal antibody MASF-IV-1 suggests that both reagents recognize the same antigenic determinant in the seroagglutination test. The serological behavior observed in the current study along with the results obtained by others researchers6,11 leads us to propose the epitope E1037 as a novel S. flexneri group factor and to highlight the need to agree on a serotyping scheme and an updated nomenclature including the new strains described.
Nowadays, monoclonal antibody MASF IV-1 is the only available reagent for the detection of S. flexneri E1037-antigen carrier isolates, which is neither accessible for use in our country nor in most Latin American countries. Having considered that the correlation between the antibody and the AA479 antiserum was 100%, the indistinct use of one or the other would lead to obtain comparable information worldwide. We propose AA479 antiserum to be used as a novel serotype marker for the additional characterization of S. flexneri isolates in Argentina as well as in Latin America, in order to cooperate with the epidemiological surveillance of Shigella spp. in the region.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank all members of the National Network for Bacterial Diarrhea and Food Borne Pathogens for their valuable participation in epidemiological surveillance. We would like to thank Dr K. Talukder (International Centre for Diarrhoeal Disease Research, Bangladesh) for the kind supply of S. flexneri 7a.