Leishmaniasis is a major vector-borne disease triggered by an obligate intracellular protozoan parasite of the genus Leishmania and transmitted by the bite of phlebotomine female sand flies. This parasite causes a wide range of human diseases, from localized self-healing cutaneous lesions to fatal visceral infections. The aim of this study was to investigate the cytotoxic, antiproliferative, and apoptotic effects of curcumin on Leishmania major promastigotes (MHOM/SA/84/JISH) and to assess these effects on the cell cycle of promastigotes. The MTT colorimetric assay was used to evaluate the cytotoxicity and proliferation of promastigotes. Additionally, flow cytometry was used to analyze the cell cycle. The Annexin V/propidium iodide staining technique followed by flow cytometry was used to study the cell death induced by curcumin. In this study curcumin showed a potent antileishmanial effect, exhibiting cytotoxicity against L. major promastigotes. At 80μM, the survival in curcumin treated promastigotes reached 22%; however, the median lethal concentration of curcumin (LC50) was 35μM. The drug exerted its cytotoxic effect by inducing apoptosis. Curcumin-induced cell death in promastigotes reached 82.5% at 80μM concentration. In addition, curcumin delayed the cell cycle in the S-phase inhibiting cell proliferation. Thus, curcumin was shown to be effective against L. major promastigotes. Therefore, curcumin merits further research studies to demonstrate its efficacy in treating cutaneous leishmaniasis.
La leishmaniasis es una enfermedad vectorial significativa, obligadamente desencadenada por un protozoo intramacrófago del género Leishmania, que se transmite por la picadura de mosquitos flebótomos hembra. Esta especie parasitaria causa una gran variedad de enfermedades humanas: desde lesiones cutáneas localizadas autocurables hasta infecciones viscerales mortales. El objetivo de este estudio fue investigar los efectos citotóxicos, antiproliferativos y apoptóticos de la curcumina en los promastigotes de Leishmania major (MHOM/SA/84/JISH) y evaluar su efecto en el ciclo celular de este parásito. Se utilizó el ensayo colorimétrico MTT para evaluar la citotoxicidad y la proliferación de los promastigotes. Además, se utilizó la citometría de flujo para analizar su ciclo celular. Se empleó la técnica de tinción de ioduro de propidio/anexina V y, posteriormente, citometría de flujo para estudiar la muerte celular inducida por la curcumina. En este estudio, la curcumina reflejó un potente efecto leishmanicida. También exhibió citotoxicidad frente a los promastigotes de L. major. A una concentración de 80μM, la supervivencia de los promastigotes tratados con curcumina fue del 22%; en tanto que la concentración letal media (LC50) fue de 35μM. El fármaco produjo su acción parasiticida mediante la inducción de apoptosis. La muerte celular inducida por curcumina en los promastigotes fue del 82,5% a una concentración de 80μM. Además, la curcumina demoró el ciclo celular en la fase S e inhibió así la proliferación celular. La curcumina demostró efectividad frente a los promastigotes de L. major y, por tanto, consideramos que los estudios deberían continuar a fin de demostrar su efectividad para tratar la leishmaniasis cutánea.
Leishmaniasis is a vector-borne disease triggered by a heterogeneous group of protozoa that belongs to the Leishmania genus15. This disease constitutes an emergent threat, with elevated morbidity and mortality levels, and has three main forms: cutaneous, mucocutaneous, and visceral5. Leishmaniasis is a common condition that affects 12 million people worldwide with an approximate 1–2 million new cases arising annually2. The cutaneous form is expected to affect around 10 million people39. The broad geographical distribution is mainly due to the constant increase in the risk factors of leishmaniasis, including migration, environmental changes, deforestation, urbanization, immunosuppression, and malnutrition. Consequently, leishmaniasis represents a potential threat to several areas27. The lack of effective vaccines, the problems with vector control and the development of drug resistance have also raised the incidence of this disease5. The most commonly used medications for leishmaniasis treatment are pentavalent antimonials, amphotericin B, paromomycin, and pentamidine, which have severe side effects, require high doses for long periods of time, and are administered parenterally28. An effective drug would be beneficial to overcome the complications caused by leishmaniasis chemotherapy if it delivers safe care at a reasonable cost. Recently, phytotherapy has received considerable attention as an alternative to chemotherapy in the treatment of parasitic diseases9.
Curcumin (diferuloylmethane) is one of the most commonly characterized phytochemicals. It is a yellow natural product extracted from the rhizome of Curcuma longa (turmeric) that is used as food spice and colorant. Curcumin is a polyphenolic, non-toxic and pharmacologically active substance that has antioxidant, anti-inflammatory and antiparasitic activities. For centuries, it has been used as a therapeutic agent against different diseases21. Antiprotozoal activities of curcumin have also been identified both in vitro and in vivo for Plasmodium12, Leishmania13,22,34, Trypanosoma26, and Giardialamblia29. Curcumin was able to reduce parasitemia by 80–90% in mice infected with Plasmodiumberghei33. Curcumin has various anticancer properties including the inhibition of cell proliferation and angiogenesis as well as cell death induction in tumor cells1. Alternative treatments, including dietary supplements and herbal remedies, are increasingly of interest today. The multiple health benefits and medicinal properties of curcumin attributed to its antioxidant and anti-inflammatory effects have received worldwide attention. Curcumin has a long established safety record, it has been accepted as a safe compound without toxic effect by the FDA. The allowable daily intake of curcumin is 0–3mg/kg body weight19. Up to 12g/day of curcumin consumption has been shown to have no harmful effect on individuals14. In the present study an attempt was made to evaluate curcumin's leishmanicidal, antiproliferative and apoptotic effect as well as its capacity to delay the cell cycle in the S-stage on Leishmania major promastigotes.
Materials and methodsParasite cultureLeishmania major promastigotes (MHOM/SA/84/JISH)16 of the Saudi strains were cultured in a complete medium consisting of RPMI-1640 (GIBCO, New York, USA) supplemented with 2mM l-glutamine, 10mM HEPES, 24mM NaHCO3, 100U/ml penicillin, 100μg/ml streptomycin and 10% heat-inactivated fetal bovine serum (Sigma Aldrich, St. Louis, MO, USA). Parasites were incubated at 26°C in a 5% CO2 atmosphere (Heracell-1501 CO2 Incubator, Thermofisher). Promastigotes were collected and used to evaluate the antileishmanial activity of curcumin.
Cell viability measurements by the MTT assayDifferent concentrations of curcumin (Sigma Aldrich, St. Louis, MO, USA) were prepared in dimethyl sulfoxide (DMSO). Exponential-phase L. major promastigotes in cultured media (1.5×106/ml) were seeded into 96-well plates and treated at the desired concentrations of the drug at final concentrations of 20, 40, 60, 80μM. DMSO was used to treat the control wells. Blank only wells contained media. All tests were performed in triplicate. Plates were incubated at 26°C for 48h in 5% CO2. A modified MTT colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma Aldrich, St. Louis, MO, USA) was performed to detect promastigote viability. The MTT reagent (250μg/ml) was added to each well and the plates were incubated at 26°C for 4h. DMSO was added to dissolve the formazan crystals. The amount of cleaved tetrazolium salts to formazan, which is directly correlated with the number of metabolic active cells in culture, was quantified at 540nm of absorbance using the enzyme-linked immunosorbent assay (ELISA) reader (Synergy LX Multi-Mode Reader, Bioteck), according to Mosmann25. Cell viability was calculated on the basis of the equation: absorbance of the treated sample/absorbance of the control cells×100. All values are means of triplicate wells. Fifty percent (50%) inhibitory concentration (LC50) according to Branquinha and coworkers6 (a drug concentration that reduces the cell growth rate by 50%) was determined from the dose-responsive curve. Results are expressed as the means and standard deviations of three independent experiments, each performed in duplicate.
Annexin V/PI and flow cytometryPromastigotes were cultured as described before and either treated with DMSO, used as a control or tested with curcumin at 20, 40, 60, 80μM concentrations. Promastigotes were harvested after 48h and washed twice in cold PBS, then centrifuged using Heraeus™ Fresco™ 21 Microcentrifuge, Thermofisher. Promastigotes were stained with propidium iodide (PI) and Alexa Fluor 488 Annexin V (Life technologies, Germany) in accordance with the manufacturers’ instructions. The percentage of cells was determined by the BD FACSCalibur™ flow cytometer and the Cell Quest Pro software from Becton Dickinson (San Jose, CA, USA). Three independent experiments were performed for each promastigote culture38.
Cell proliferation measurements by the MTT assayPromastigotes were cultured and incubated as in the viability assay mentioned above. Curcumin was added to 96-well plates at a final concentration of 80μM. Plates were incubated at 26°C in 5% CO2 at different time intervals (0, 24, 48, 72h). The MTT reagent was added and the absorbance was measured at 540nm by the enzyme-linked immunosorbent assay (ELISA) reader. The results were expressed as means and standard deviations of three independent experiments, each of which was performed in duplicate24.
Cell cycle analysis by flow cytometryPromastigotes were treated with DMSO or curcumin (80μM) and then collected and re-suspended in 1ml of Phosphate-Buffered Saline (PBS) before being fixed with a drop of 3ml of 100% methanol. Fixed promastigotes were centrifuged, re-suspended at 50μl of RNase (1mg/ml) and incubated at room temperature for 30min, followed by the addition of 1ml of 0.1mg/ml of propidium iodide (PI)11. The DNA content of the cells was analyzed by flow cytometry (Becton Dickinson). The percentage of promastigotes in various cell-cycle phases was determined using the Cell Quest software (Becton Dickinson).
Statistical analysisData were presented as mean and standard deviation using SPSS-17 statistical software. Comparisons between groups were rendered with the Student's t-test. LC50 was obtained through an exponential regression data analysis using the Sigma-plot computer software.
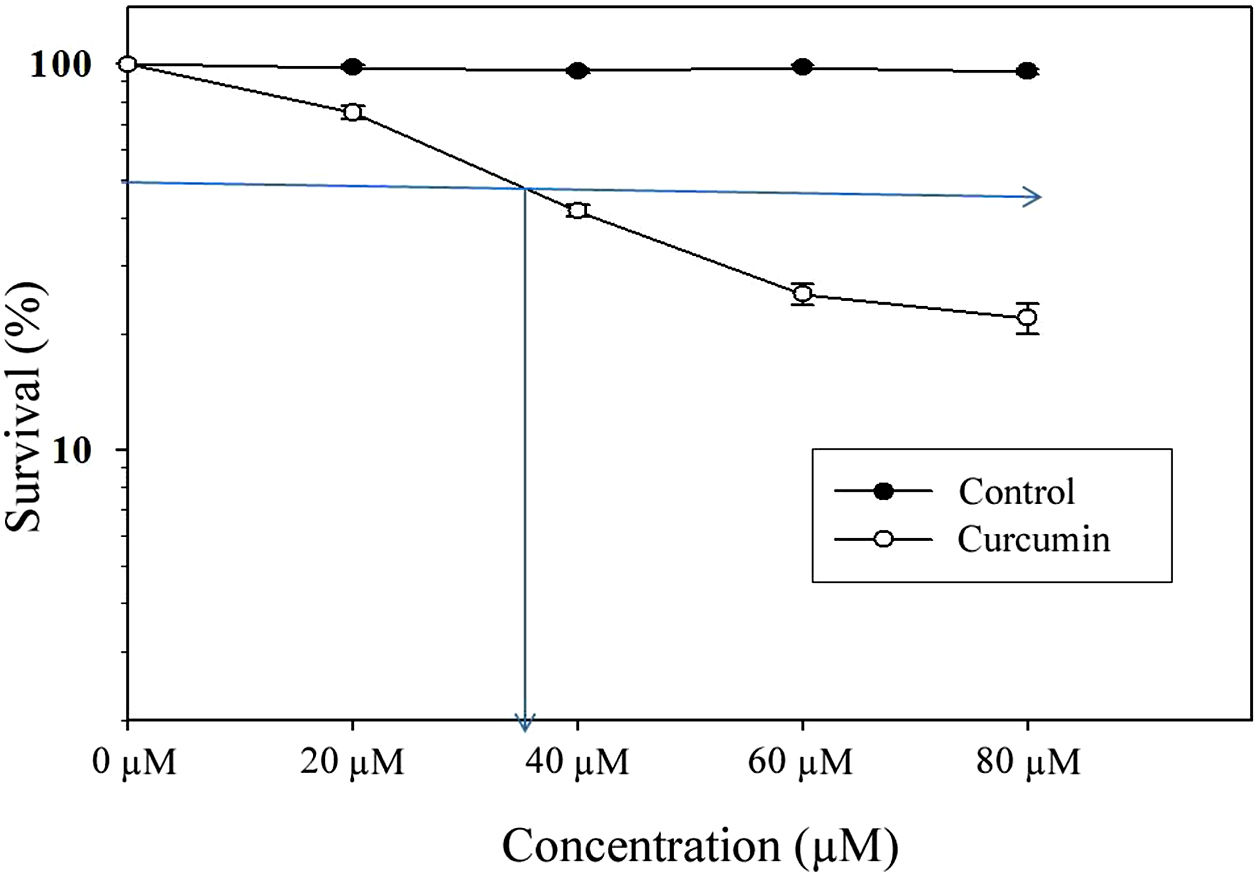
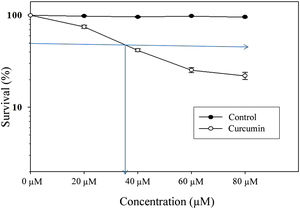
ResultsCytotoxic effect of curcumin on L. major promastigotesThe MTT assay was used to investigate the cytotoxic effects of curcumin on Leishmania major promastigotes. Figure 1 shows the dose-dependent effect of curcumin on L. major promastigotes. The survival percentage of promastigotes of the curcumin-treated groups was statistically compared to the non-treated control group. The results showed that promastigotes were highly sensitive to curcumin, particularly when the dose was increased. The median lethal curcumin concentration (LC50) was 35μM (Fig. 1). In the absence of curcumin, L. major promastigotes survival was 100%, whereas at 20μM curcumin, promastigotes survival was 75% (Fig. 1). Survival decreased to 41.5% at 40μM curcumin. At 60μM, there was a sharp drop in promastigote survival, reaching 25.3%; however, at 80μM, survival in curcumin-treated promastigotes decreased to 22% (Fig. 1). The difference in survival between curcumin-treated and non-treated promastigotes was highly significant (p<0.001) (t=50.7).
Cytotoxic effect of curcumin on L. major promastigotes. Exponentially growing promastigotes were cultured in 96-well plates and treated at the indicated concentrations of curcumin for 48h. Cell death was analyzed using the MTT assay. The arrows indicate LC50. Error bars represent standard deviations of at least three different experiments.
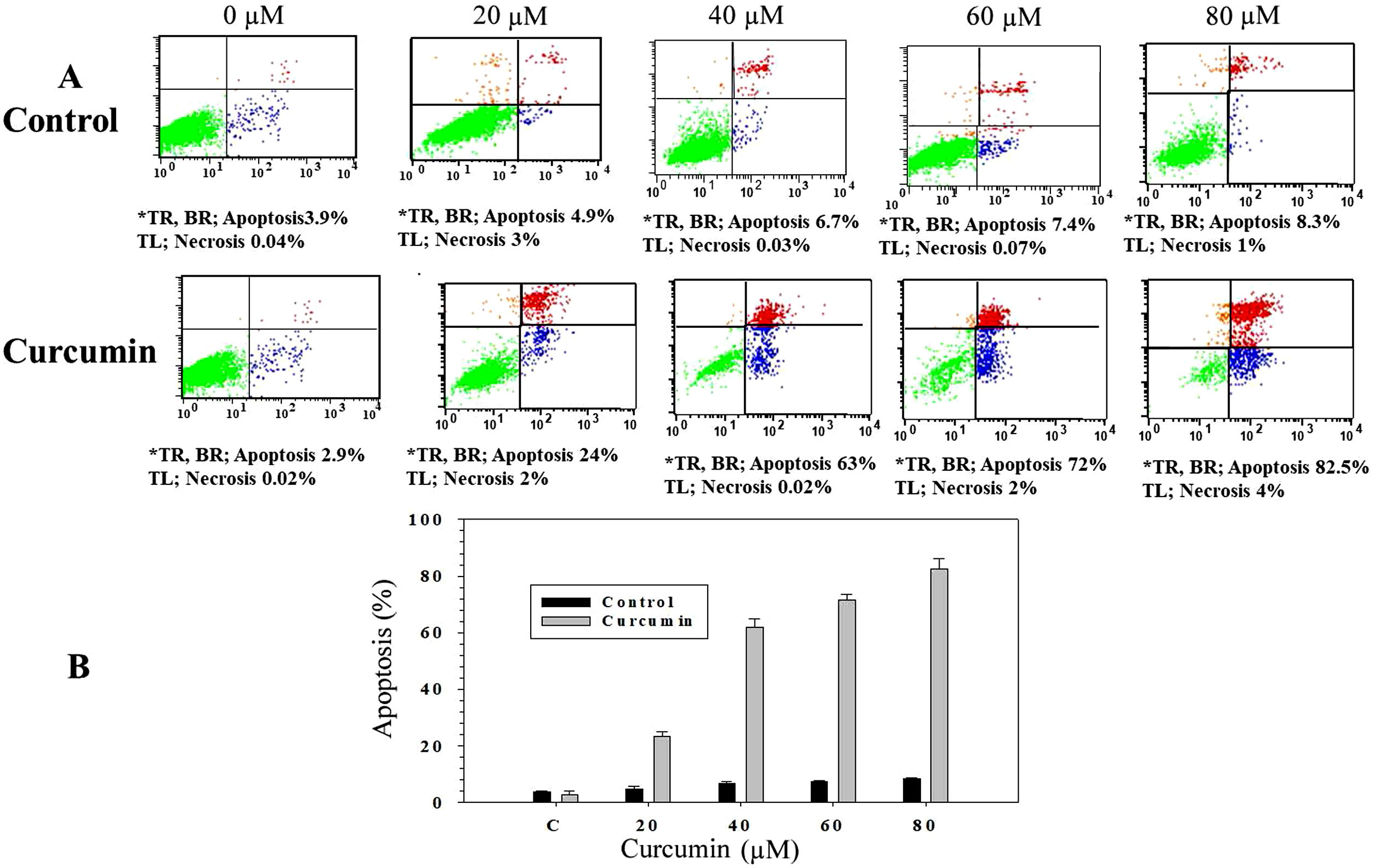
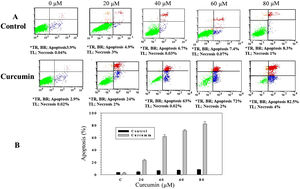
In order to study whether the mechanism of cell death triggered by curcumin is due to apoptosis or necrosis, an Annexin V/propidium iodide (PI) staining technique followed by flow cytometry was used. L. major promastigotes were treated at different curcumin concentrations for 48h and then stained and sorted. Fig. 2A shows four groups of cells, viable cells that excluded both Annexin V and PI (Annexin V−/PI−), bottom left; early apoptotic cells that were only stained with Annexin V (Annexin V+/PI−), bottom right; late apoptotic cells that were stained with both Annexin V and PI (Annexin V+/P+), top right; and necrotic cells that were only stained with PI (Annexin V−/PI+), top left. Following the deduction of the proportion of spontaneous apoptosis, the proportion of apoptosis was assumed to be the amount of both early and late apoptosis. Figure 2A and B, exhibits the cytotoxicity of curcumin against L. major promastigotes. Importantly, curcumin induced death by apoptosis, this impact increased in a dose-dependent manner. The proportion of apoptotic promastigotes at 20μM was approximately 24% (Fig. 2A and B). The proportion was 63% in response to 40μM curcumin; however, curcumin-induced apoptosis was 72% at 60μM. The maximum proportion of cell death (82.5%) was achieved when promastigotes were treated with 80μM curcumin (Fig. 2A and B).
Curcumin triggers apoptosis in L. major promastigotes. Promastigotes were either shamtreated or challenged at the indicated concentrations of curcumin for 48h and then cell death was analyzed using the Annexin V/PI flow cytometry assay. (A) Charts indicating the proportion of apoptotic and necrotic cells. (B) Histogram showing the proportions of apoptotic cells. The difference in apoptosis between curcumin-treated (different doses) and non-treated promastigotes was highly significant (p<0.001). *TR, top right; BR, bottom right; TL, top left.
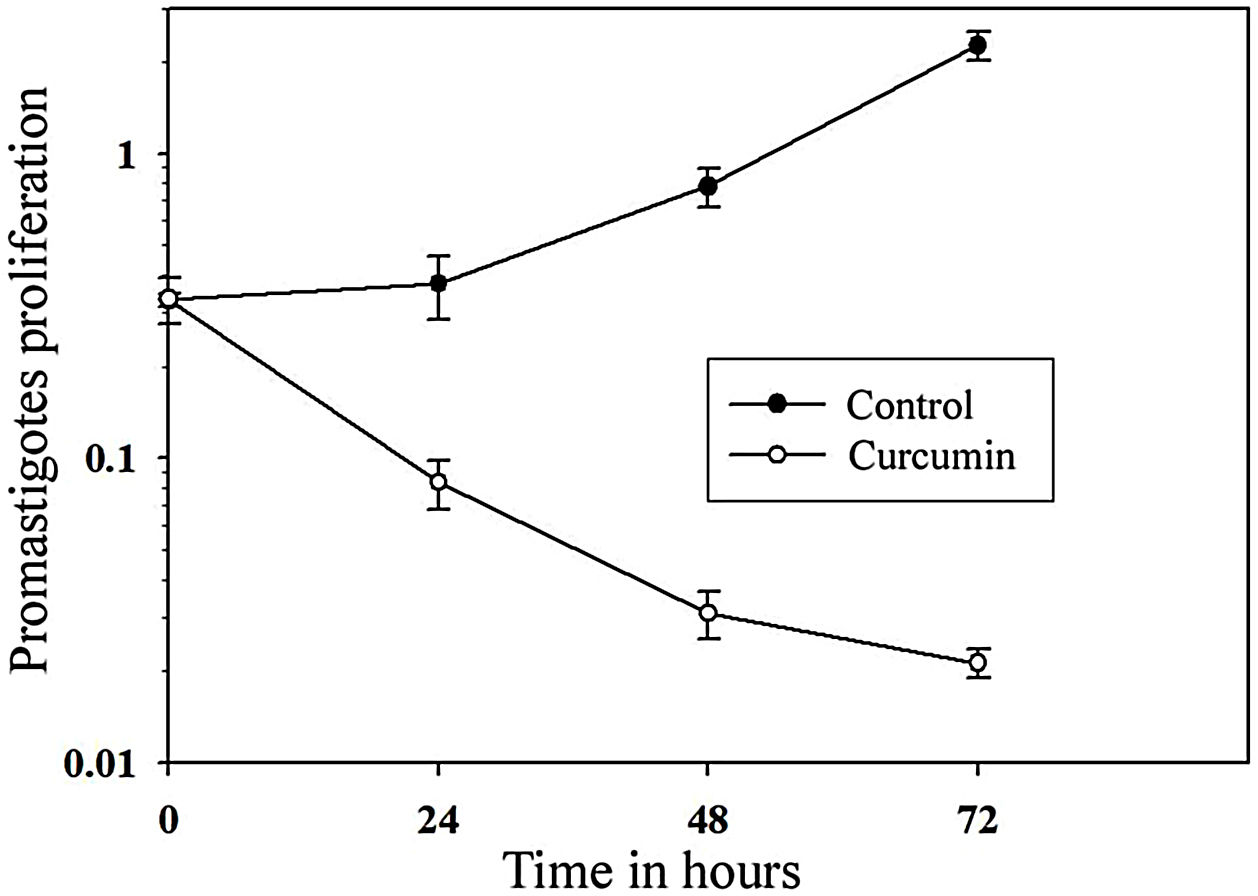
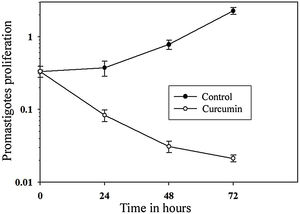
The impact of curcumin on L. major promastigote proliferation was analyzed using the MTT cell proliferation assay. Figure 3 shows that while the control with non-treated promastigotes continued to proliferate in a time-dependent manner, the number of curcumin-treated promastigotes decreased sharply only after 24h of treatment and continued to decline after 72h of treatment. Comparing curcumin-treated with non-treated promastigotes in the control group, there was a highly significant difference in promastigote proliferation after 72h of treatment (p<0.001) (t=15.8) (Fig. 3).
Curcumin inhibits the proliferation of L. major promastigotes. Promastigotes were cultured in 96-well plates and were either sham-treated (DMSO) or challenged with curcumin (80μM) for the indicated periods of time, and then cell proliferation was assessed by the MTT assay. Error bars represent standard deviations of at least three different experiments.
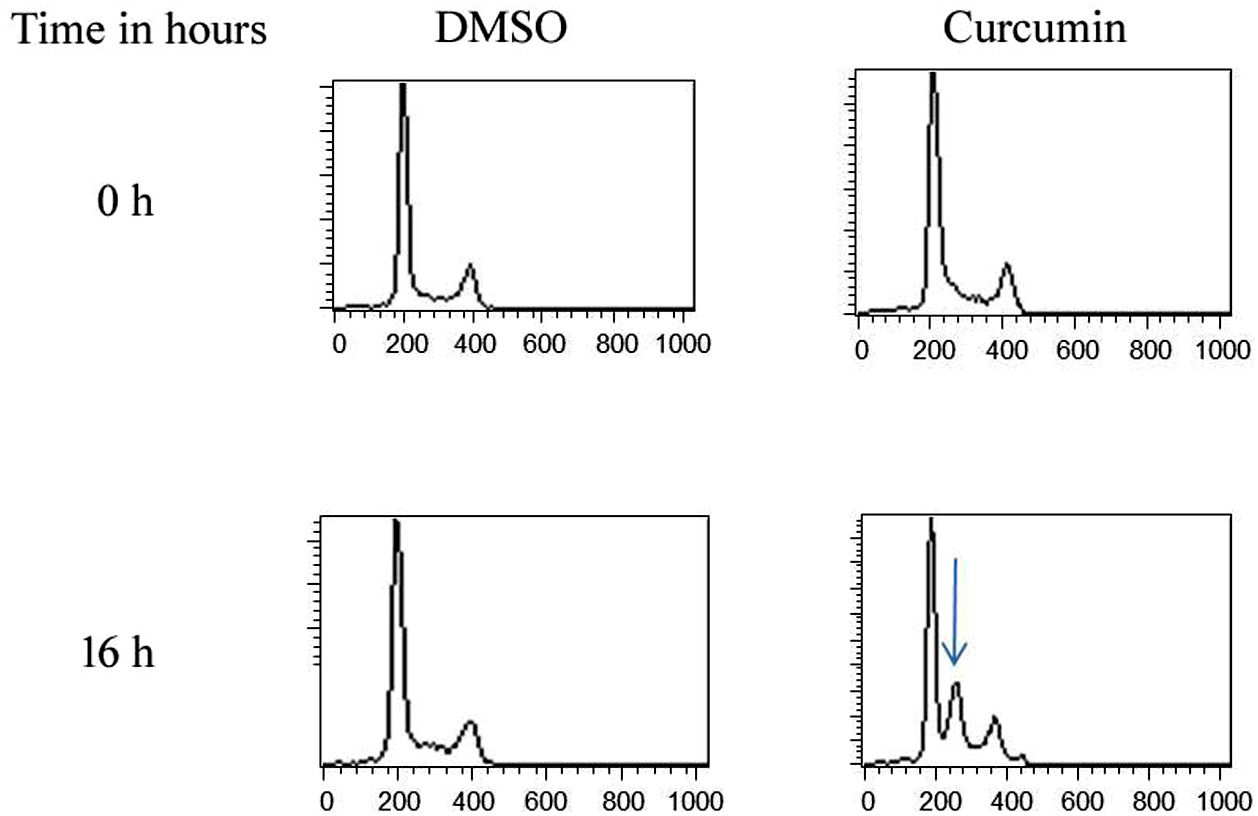
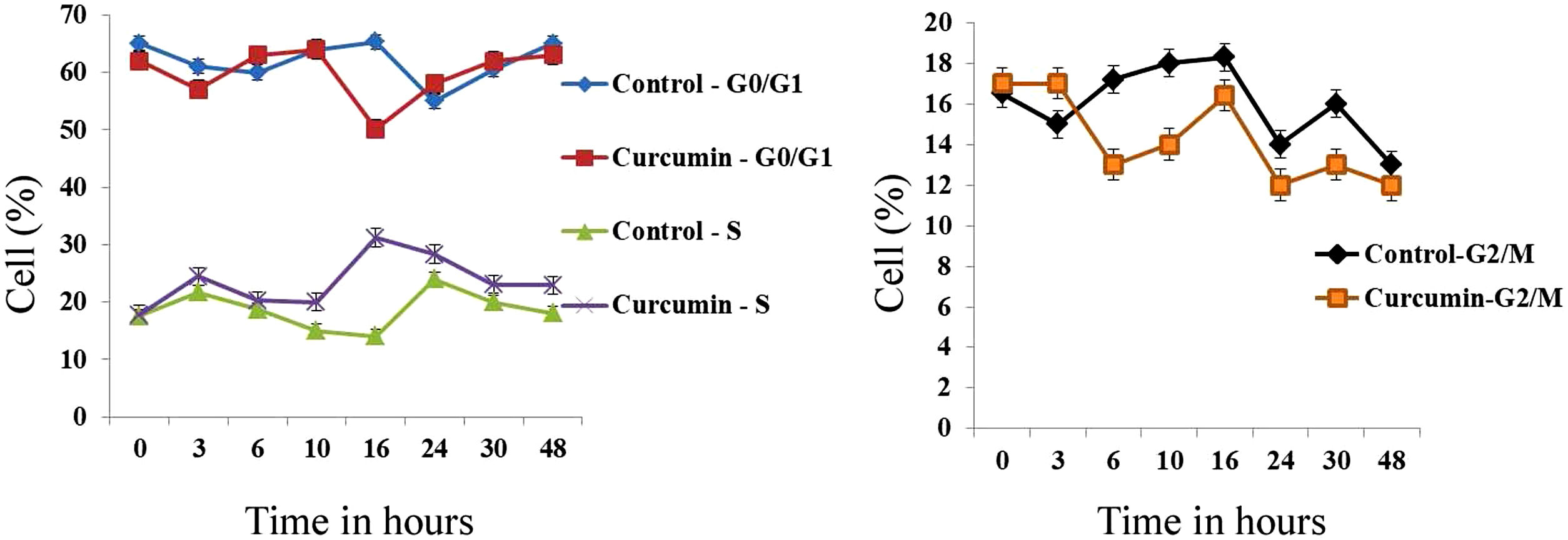
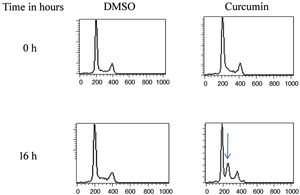
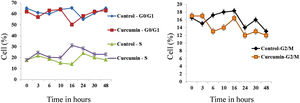
The effect of this agent on the cell cycle was investigated after demonstrating the inhibitory effect of curcumin on cell proliferation. To this end, L. major promastigotes were either sham-treated or challenged with curcumin (80μM) at various time intervals, and then cells were fixed, stained with PI. The cell cycle examined by flow cytometry (Figs. 4 and 5), showed curcumin-dependent cell accumulation in the S-phase reaching, after 16h of incubation, a maximum level of about 33% compared to 14% in the control group (Figs. 4 and 5).
Curcumin inhibits the proliferation of L. major promastigotes by delaying cell cycle at S phase. Cells were either sham-treated or challenged with Curcumin (80μM) for different time intervals. The cell cycle status was analyzed by flow cytometry. The arrow indicates the accumulated cells at S phase. The curves show the proportions of cells in various phases of the cell cycle as indicated.
Most drugs currently used for leishmaniasis have several limitations, such as high toxicity, challenging treatment schedules, and development of resistance20. Therefore, there is an urgent need for new, safe, more effective and economically feasible drugs for the treatment of leishmaniasis. Herbal products have been used for the treatment of multiple human diseases for thousands of years. Low specificity, adverse reaction and toxicity of chemotherapeutic agents are commonly observed during chemotherapy treatment. Miltefosine has not been used in this study; it has been used in Leishmania treatment in many previous studies. The main safety concerns of miltefosine are related to its effect on the mucosa of the gastrointestinal tract and its potential for teratogenicity. The gastrointestinal side effects of miltefosine include loss of appetite, fatigue, diarrhea, or a combination of these events. Many commonly reported toxic effects associated with miltefosine mainly involve the kidneys and the liver, affecting the activity of the liver, inducing elevated bilirubin, SGOT and SGPT, and causing detrimental effects on kidney function marked by elevated creatinine and serum aspartate aminotransferase30. Curcumin, a natural pharmacologically active agent, has several anti-inflammatory mechanisms that may be detrimental to effective host control of intracellular microbial infections. In our/this report, we have provided clear evidence that curcumin may constitute a potent antileishmanial agent against L. major promastigotes in vitro.
The present study shows that curcumin has a dose-dependent cytotoxic effect against L. major promastigotes. Previously, the leishmanicidal activity of curcumin was dose-dependent22,34. In the present analysis, the median lethal concentration of curcumin (LC50) was 35μM, being these results compatible with those from Kodie and coworkers which obtained an LD50 value of 37μM for curcumin activity against L. major promastigotes invitro22. In a previous report, curcumin displayed LD50 of about 4.5, 5.7, and 5.9μM against L. major, Leishmania tropica, and Leishmania donovani promastigotes, respectively, with 100% of curcumin-induced killing at 13.5μM34. The maximum parasite killing proportion achieved in this analysis was roughly 80%; however, this percentage, a drug concentration of up to 80μM was used to obtain this percentage. The difference in results could be explained by the metabolic variability during the development of Leishmania promastigotes in cultures and variations in different in vitro culture conditions. Curcumin has an outstanding safety profile. Indeed, different phase I clinical trials have shown that curcumin is safe when administered at doses up to 12g daily for 3 months21,36. In another study, the dose-dependent death of L. donovani promastigotes occurred with curcumin at concentrations between 20 and 60μM, reaching 90% when 55μM of the drug was used13.
The Annexin V/propidium iodide (PI) staining technique was used to confirm the cytotoxic nature of curcumin and to identify the death pathway that this agent triggers in L. major promastigotes. The apoptotic activity of curcumin was dose-dependent. Interestingly, the killing function of the drug is by apoptosis and the maximum necrotic death (4%) was reached when promastigotes were administered at the highest dose (80μM) of the drug. This could be explained by the fact that curcumin may have the ability to modulate numerous targets and cell signaling pathways, as well as affect gene-regulating apoptosis. In a previous report, we showed that apoptosis was triggered by curcumin via the mitochondrial pathway by downregulation of Bcl-2, a downstream anti-apoptotic effector of the Shh signaling in medulloblastoma cells17. The pharmacological efficacy of curcumin against Leishmania parasites is also unclear. One of the features of metazoan apoptosis is the lack of mitochondrial membrane capacity, which has been reported to play a key role in drug-induced death in protozoans such as Leishmania35. Curcumin-induced development of reactive oxygen species (ROS) and elevation of cytosolic calcium have been previously demonstrated, followed by exposure of phosphatidylserine to the outer plasma membrane leaflet and DNA fragmentation in L. donovani promastigotes13. Using L. donovani species, both in vitro and in vivo, leishmanicidal activity was induced by nanoformulation of curcumin alone; however, curcumin showed in vitro inhibition of both promastigotes and amastigotes when combined with miltefosine. Increased in vivo leishmanicidal activity, along with increased production of toxic reactive oxygen and nitrogen metabolites, was also caused by combination therapy37. The bioavailability of curcumin was enhanced when nanoparticles were administered. At the subcurative dose, mitefosine leishmanicidal effect was supported by poly (lactide-co-glycolide) loaded with curcumin (nanocurcumin) through immunomodulation. No hepatotoxic or nephrotoxic effects were observed with nanocurcumin monotherapy or in combination with miltefosine37.
The apoptotic pathway in Leishmania is still unknown; however, the proteins involved in apoptosis are also unknown. Leishmania lacks the mammalian key proteins involved in apoptosis32. In L. major, although LmjMCA (a cysteine peptidase) has different substrate specificities, it shares similarities with caspases. In a recent report, three pathways involved in apoptosis have been identified in L. major: the first pathway through which miltefosine mediated LmjMCA activation; the second pathway through which LmjMCA was inhibited by amphotericin B, curcumin and H2O2 and the third apoptotic pathway that was induced by pentamidine and considered to be independent of LmjMCA. A previous report also found that LmjMCA plays a role similar to caspases in both cell death and autophagy in L.major8. The cell death effector protein LmjHYD36 overexpression was induced by curcumin in both the L. major wild-type strain and the LmjMCA-deficient strain4.
It was suggested that different apoptotic pathways in Leishmania should be involved. Non-caspase proteases, such as calpains, have been involved in Leishmania cell death7; however, cathepsin B-like enzyme LmjCPC has also been detected in different domains18. In a previous study, trans-dibenzalacetone (DBA), a synthetic curcumin analog demonstrated leishmanicidal efficacy against L. donovani amastigotes. In addition to cell cycle arrest at the G0/G1 phase, it exhibited antiproliferative effects with increased concentration of cytosolic calcium and dissipation of mitochondrial membrane potential. DBA also induced inhibition of the trypanothione/trypanothione reductase (TR) system of Leishmania cells10. Using curcumin analog DBA, L. donovani enters the late apoptotic stage without passing through the early apoptotic phase; however, in our study curcumin showed both early and late apoptosis in L. major and this could be explained by the different modes of action of the two different therapeutic molecules or by the different apoptotic pathways of the different Leishmania strains. Curcumin and miltefosine increased L. major apoptosis by overexpressing LmjF.22.0600, a potential acetyltransferase that is conserved between different Leishmania species3.
In a previous research, the photodynamic activity of curcumin on L. major and Leishmania braziliensis promastigotes was examined. The trypan blue test showed that curcumin photodynamic treatment had a significant effect on the viability and morphology of parasites. The rise in curcumin concentration in both the nucleus and kinetoplast of Leishmania clarified the increased mitochondrial activity, which suggested apoptosis31. In addition, curcumin is a potent inhibitor of promastigote proliferation by delaying the S-phase cell cycle. The inhibition was very sharp after just 24h of treatment. It has been previously shown that curcumin induced cell cycle arrest at the G2/M phase in L. donovani promastigotes13. Curcumin may inhibit certain biological reactions in mammalian cells, for example, we have previously shown that curcumin inhibited cell proliferation and triggered the G2/M phase of cell-cycle arrest in medulloblastoma cells17. Curcumin has also been reported to interrupt cell cycle progression in colon cancer cells23.
Collectively, these data show that curcumin has potent leishmanicidal and antiproliferative properties against L. major promastigotes. It triggers cell death mainly by apoptosis and thus warrants further investigations of its potential use as a chemotherapeutic agent against cutaneous leishmaniasis. Future studies on intracellular amastigotes and animal models are recommended. Molecular studies should be conducted to further investigate the apoptotic pathway triggered by this molecule.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors acknowledge financial support from the researchers supporting project number (RSP-2020/111). King Saud University, Riyadh, Saudi Arabia.