Cleaning and disinfection represent the most important activities associated with the elimination of dirt and microorganisms at food processing plants. Improper procedures may lead to cross contamination of food leading to its spoilage or even the transmission of foodborne pathogens. Several strategies have been used in order to achieve a good disinfection of surfaces and products; nevertheless, microbial resistance to common-use-products has developed lately. Due to this fact, the development of new non-toxic-food compatible chemical agents that reduce the impact of foodborne pathogens and spoilage causing microorganisms is desirable for the food industry. The objective of the present study was to evaluate the antimicrobial activity of different sodium and potassium salts of aliphatic and aromatic carboxylic acid on the growth of common food spoilage and pathogenic microorganisms. Growth curves were determined for Leuconostoc mesenteroides, Lactobacillus plantarum, Enterococcus faecalis, Candida albicans, Pseudomonas aeruginosa, Salmonella Enteritidis, and Listeria monocytogenes in contact with different concentrations of carboxylic acid salts. The inhibitory effect of both aliphatic and aromatic carboxylic acid salts, in accordance with concentration levels, was 100>50>25mg/ml. The inhibitory effect of aliphatic salts was butanoic>hexanoic> octanoic>decanoic and, benzoic>gallic>caffeic acid salts for aromatic salts. In general, sodium salts were more inhibitory than potassium salts (p≤0.05).
La limpieza y la desinfección representan las actividades más importantes asociadas a la eliminación de suciedad y microorganismos de las plantas procesadoras de alimentos. El uso de procedimientos incorrectos puede llevar a la contaminación cruzada de los alimentos y, por ende, al deterioro de estos o a la transmisión de patógenos de origen alimentario. Se han desarrollado varias estrategias con el fin de obtener una buena desinfección de superficies y productos; no obstante, ha aparecido resistencia microbiana frente a productos de uso común. Debido a esto, el desarrollo de agentes químicos no tóxicos capaces de reducir el impacto de patógenos de origen alimentario y microorganismos causantes de deterioro es deseable para la industria alimentaria. El objetivo de este estudio fue evaluar la actividad antimicrobiana de diferentes sales de sodio y potasio de ácidos carboxílicos alifáticos y aromáticos sobre algunos microorganismos patógenos y asociados a deterioro alimentario, analizando su impacto sobre el crecimiento. Se determinaron las curvas de crecimiento de Leuconostoc mesenteroides, Lactobacillus plantarum, Enterococcus faecalis, Candida albicans, Pseudomonas aeruginosa, Salmonella enteritidis y Listeria monocytogenes en presencia de diferentes concentraciones de las sales de ácidos carboxílicos. El efecto inhibitorio de las sales de ácidos carboxílicos alifáticos y aromáticos, según su nivel de concentración, se ordenó del siguiente modo: 100mg/ml>50mg/ml>25mg/ml. El efecto de las sales de ácidos alifáticos siguió el orden butanoico>hexanoico>octanoico>decanoico, en tanto que las de ácidos aromáticos se ordenó del siguiente modo: benzoico>gálico>cafeico. En general, las sales de sodio fueron más inhibitorias que las de potasio (p ≤ 0,05).
Cleaning and disinfection represent the most important activities associated with the elimination of dirt and microorganisms at food processing plants. Improper procedures may lead to cross contamination of food leading to its spoilage or even the transmission of foodborne pathogens4. Several strategies have been used in order to achieve a good disinfection of surfaces and products; nevertheless, microbial resistance to common-use-products has developed lately, even though microorganisms vary greatly in their resistance to chemical germicides and sterilization processes7. Due to this fact, the development of new non-toxic-food compatible chemical agents that reduce the impact of foodborne pathogens and spoilage-causing microorganisms is desirable for the food industry5,17.
Recently, different researchers have described the inhibitory action of short chain organic acids and/or their salts on the growth of bacteria and molds25, including pathogens22,24. These products are generally recognized as safe (GRAS) compounds frequently used as chemical decontaminants18.
Acetate, lactate and citrate sodium salts have shown an inhibitory effect on the growth of some food spoilage bacteria and antimicrobial activity against foodborne pathogens, including Staphylococcus aureus and Yersinia enterocolitica11, Listeria monocytogenes15, Escherichia coli11,12, as well as Clostridium botulinum1. Moreover, a limited antimicrobial capacity has been reported for some organic acid salts against lactic acid bacteria during meat spoilage6,14.
Because of the association of salt consumption with the risk of hypertension, health promoters advise a lower intake of this compound; nevertheless, there has been controversy regarding the effect of salt removal or reduction on the shelf life and safety of food products. Potassium salt intake is actually promoted by the World Health Organization (WHO), and the new guidelines advise adults to consume less than 5g of salt and at least 3.510mg of potassium per day in order to reduce the risk of high blood pressure23.
Potassium salts have also been tested for their antimicrobial effect. Some researchers have described inhibitory effects on pathogenic bacteria, including L. monocytogenes, Vibrio parahaemolyticus and Clostridium perfringens1; nevertheless, there is still controversy over the subject.
The objective of the present study was to evaluate the inhibitory effect of different sodium and potassium organic salts on the growth of common food spoilage and pathogenic microorganisms including Leuconostoc mesenteroides, Lactobacillus plantarum, Enterococcus faecalis, Candida albicans, Pseudomonas aeruginosa, Salmonella Enteritidis and L. monocytogenes.
Materials and methodsGeneral chemical methodsAll glassware and syringes were dried in an oven at 140°C overnight and flushed with nitrogen immediately prior to use. Tetrahydrofuran (THF) was refluxed and freshly distilled from potassium/benzophenone ketyl under a nitrogen atmosphere.
The reagents used included sodium hydride, potassium hydride, butanoic acid, hexanoic acid, octanoic acid, decanoic acid, benzoic acid, caffeic acid and gallic acid. All the reagents were used without any further purification.
Preparation of sodium carboxylic acid saltsNaH (60% in mineral oil) was poured (0.728g) into a round bottom flask and kept under a nitrogen atmosphere. Dry THF (20ml) was added to the flask and stirred; after a few minutes NaH was allowed to settle. The supernatant liquid was removed with a double-tipped cannula under nitrogen and the procedure repeated. The remaining THF was evaporated under vacuum to obtain a dry white solid (0.542g, 22.6mmol NaH).
Dry THF (20ml) was added to the pure NaH obtained as above, and the temperature lowered to 0°C. Butyric acid (1.99g, 22.6mmol), dissolved in THF (20ml), was added dropwise with stirring in about 15min. The ice bath was removed and the suspension stirred for 18h under a nitrogen atmosphere. The solvent was eliminated in a rotary evaporator and the sodium salt was obtained as a white solid.
Potassium salts were prepared using KH instead of NaH, using the above procedure.
Growth curve determinationType cultures of L. mesenteroides (ATCC 8293-2), L. plantarum (ATCC 14917), E. faecalis (ATCC 29212), C. albicans (ATCC 10231), P. aeruginosa (ATCC 15442), Salmonella Enteritidis (ATCC 13076) and L. monocytogenes (ATCC 19116) were kindly supplied by the Bacteriology Lab, Faculty of Microbiology, Universidad de Costa Rica.
Suspension preparationEach microorganism was inoculated into trypticase soy broth (TSB)+yeast (Oxoid®) and cultured at 37°C until the desired concentration was attained. The bacterial suspension to be cultured was equivalent to 0.5 McFarland standard, (1.5×108CFU/ml).
Bactericidal assays96 well tissue culture microtiter plates (Nalge, Nunc International, Rochester, NY) were used for each experiment. The assay mixture consisted of 50μl TSB (trypticase soy broth)+yeast (Oxoid®) for all strains evaluated except L. mesenteroides and L. plantarum where MRS (Man, Rogosa and Sharpe) broth (Oxoid®) was used. Fifty (50) microliters of each bacteria suspension was used as well as 50μl of the chemical agent at the different concentrations to be tested (25, 50 and 100mg/ml). Seven wells were used as positive growth control, one well was used as negative control (no bacteria added). For each bacteria tested, 4 wells of the microtiter plate were used. The first well was used to evaluate the 25mg/ml final concentration of each chemical agent, the second well for the 50mg/ml concentration and the third one for the 100mg/ml concentration. The fourth well was used as positive growth control of the microorganism.
Growth curves were determined using the Biotek Synergy HT multi detection reader (Vermont, US). Protocol followed included 600nm readings every 30min for 24h at 37°C incubation temperature.
Data analysisAll experiments were performed in triplicate. Data was analyzed using the SPSS-PC program (Statistical Package for the Social Sciences) with a 95% (p≤0.05) confidence level.
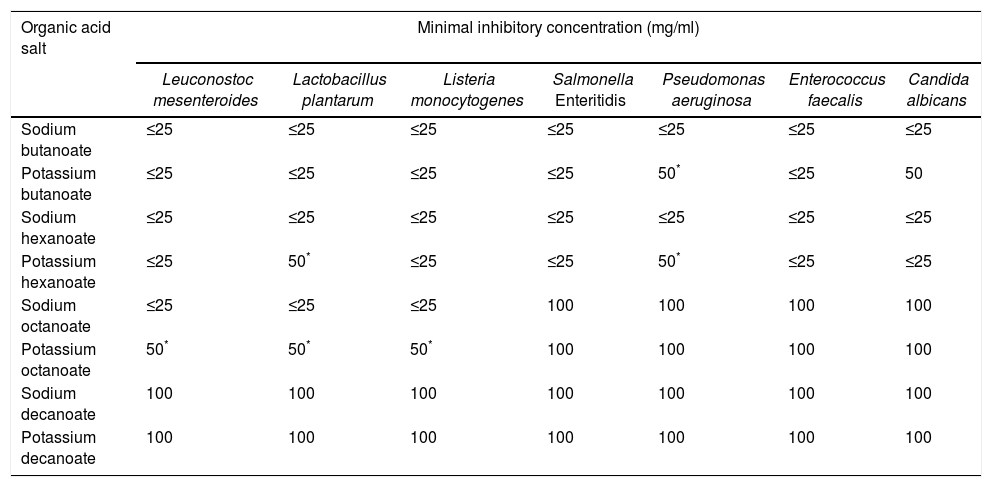
ResultsThe antimicrobial activity of different sodium and potassium salts of aliphatic carboxylic acid was evaluated in this study (Table 1). Differences (p≤0.05) between the antimicrobial activity of sodium and potassium salts were observed for some of the microorganisms tested.
MICs of the different aliphatic salts tested against seven different microorganisms
| Organic acid salt | Minimal inhibitory concentration (mg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| Leuconostoc mesenteroides | Lactobacillus plantarum | Listeria monocytogenes | Salmonella Enteritidis | Pseudomonas aeruginosa | Enterococcus faecalis | Candida albicans | |
| Sodium butanoate | ≤25 | ≤25 | ≤25 | ≤25 | ≤25 | ≤25 | ≤25 |
| Potassium butanoate | ≤25 | ≤25 | ≤25 | ≤25 | 50* | ≤25 | 50 |
| Sodium hexanoate | ≤25 | ≤25 | ≤25 | ≤25 | ≤25 | ≤25 | ≤25 |
| Potassium hexanoate | ≤25 | 50* | ≤25 | ≤25 | 50* | ≤25 | ≤25 |
| Sodium octanoate | ≤25 | ≤25 | ≤25 | 100 | 100 | 100 | 100 |
| Potassium octanoate | 50* | 50* | 50* | 100 | 100 | 100 | 100 |
| Sodium decanoate | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Potassium decanoate | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
The inhibitory effect of the aliphatic acid salts according to concentration levels was 100>50>25mg/ml, and according to chemical nature was butanoic>hexanoic>octanoic>decanoic acid salts. The comparison of ions shows that sodium salts have a higher antimicrobial effect than potassium salts.
L. mesenteroides was inactivated by sodium and potassium butanoate and sodium and potassium hexanoate at a minimal inhibitory concentration (MIC) of 25mg/ml. However, significant differences between sodium (MIC of 25mg/ml) and potassium (MIC of 50mg/ml) salts were observed in the case of the octanoic salt (Table 1). A MIC of 100mg/ml was observed for both decanoic acid salts. Similar results were observed for L. plantarum.
Most of the antimicrobials were effective against L. monocytogenes (MIC of 25mg/ml) with the exception of the octanoic and decanoic acid salts, where higher concentrations (50 and 100mg/ml) were necessary to observe complete inhibition. Salmonella Enteritidis, P. aeruginosa, E. faecalis and C. albicans presented all higher MICs for sodium and potassium octanoate and decanoate salts (100mg/ml) as well. Furthermore, P. aeruginosa presented higher MICs for potassium salts (50mg/ml) than for sodium salts (25mg/ml) when butanoate and hexanoate salts were evaluated.
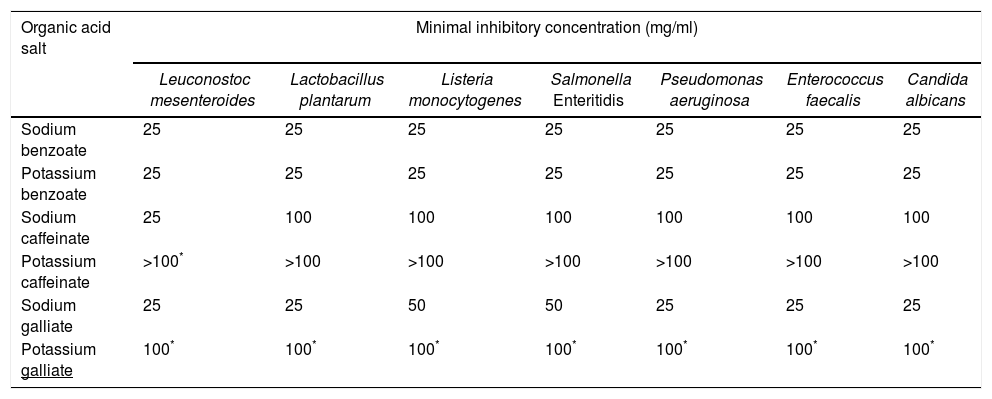
The results of the antimicrobial activity of different aromatic sodium and potassium salts are presented in Table 2. There were statistical differences (p≤0.05) between the antimicrobial activity of sodium salts compared to potassium salts.
MICs of the different aromatic salts tested against seven different microorganisms
| Organic acid salt | Minimal inhibitory concentration (mg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| Leuconostoc mesenteroides | Lactobacillus plantarum | Listeria monocytogenes | Salmonella Enteritidis | Pseudomonas aeruginosa | Enterococcus faecalis | Candida albicans | |
| Sodium benzoate | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Potassium benzoate | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Sodium caffeinate | 25 | 100 | 100 | 100 | 100 | 100 | 100 |
| Potassium caffeinate | >100* | >100 | >100 | >100 | >100 | >100 | >100 |
| Sodium galliate | 25 | 25 | 50 | 50 | 25 | 25 | 25 |
| Potassium galliate | 100* | 100* | 100* | 100* | 100* | 100* | 100* |
The antimicrobial effect of all aromatic salts tested according to concentration levels was 100>50>25mg/ml, and the antimicrobial effect according to chemical nature was benzoic>gallic>caffeic salts. The comparison of ions shows that sodium salts have the same activity as potassium ions for benzoic salts; however, a higher antimicrobial activity was observed for the sodium salts (p≤0.05) in the case of gallic and caffeic acids. All microorganisms tested presented a MIC of 25mg/ml for sodium and potassium benzoates. In the case of the sodium gallic salt, MICs of 25–50mg/ml were observed for all the microorganisms and higher concentrations of caffeic acid sodium salt (100mg/ml) were necessary to accomplish complete inhibition of all the microorganisms with the exception of L. mesenteroides (25mg/ml). MIC for potassium galliate was of 100mg/ml for all the microorganisms evaluated and the potassium salt of caffeic acid did not inhibit any of the microorganism tested (Table 2).
DiscussionFood product contamination can occur through different environmental routes, including air, water, people and even surfaces. The latter route is the most important one to control for any sanitation program20.
The increase in antimicrobial resistance has been attributed to the inadequate use of antimicrobial compounds and the transference of resistance genes within and between strains3. The development of novel, non-toxic-food compatible chemical products could help to reduce the presence of pathogens and spoilage microorganisms from environments, including surfaces, equipment and even workers’ hands.
The results obtained in this work for sodium and potassium salts of aliphatic carboxylic acid show that the antimicrobial activity of these compounds is related to the number of carbon atoms. High antimicrobial activity was observed for compounds with shorter carbon chains such as butanoate and hexanoate. Raftari et al.16 reported that the lethal effect of organic acids increases with chain length until a cut off phenomenon occurs. Kim et al.9, and Kubo et al.10 reported similar results for phenolic acids and for alkenals, respectively. However, other reports in the literature established that short and medium chain fatty acid salts were more germicidal at lower pH values than long chain molecules21. In our study, the average pH value of the experimental matrix (TSA or MRS broth) was 5.9. Lower antimicrobial activity of long chain fatty acid salts at acidic conditions could be correlated with a decrease in solubility21. These results indicate that short chain fatty acid salts could be efficient antimicrobials in food products with an acidic pH.
Other studies have also reported the antimicrobial activity of organic acid salts. Nakai et al. describe a MIC for L. plantarum of 11.4mg/ml using caproic acid, coinciding with the value reported in this study for sodium and potassium hexanoate13. The inhibition of these bacteria with different organic acid salt treatments, including sodium acetate, sodium lactate and sodium citrate has been reported in sliced salmon8.
The highest MICs were found in Pseudomonas. The MIC obtained with octanoate and decanoate sodium and potassium salts was of 100mg/ml or higher; nevertheless smaller chain salts presented MICs varying from 25 to 50mg/ml. L. plantarum and P. aeruginosa showed a decreased susceptibility to potassium salts even with the short chain fatty acids. These data indicate that the antimicrobial effect of fatty acid salts is dependent on the type of microorganism.
In the case of foodborne pathogens, Salmonella Enteritidis and L. monocytogenes growth was prevented or inhibited by all the salts tested. Raftari et al.16 have also described a significant decrease in populations of Salmonella typhimurium and L. monocytogenes after spraying meat tissues with formic, lactic, acetic and propionic acid. Same behavior is described by Silva et al. when they tested sodium lactate and sodium propionate, combined with sodium acetate in soft cheeses19. Sodium and potassium salts of fatty acids could be a practical option to control both spoilage and pathogenic microorganisms in different food products.
Phenolic products have a basic skeleton; nevertheless the number and position of the hydroxyl groups on the aromatic ring, as well as the type of substituents cause significant changes in their properties25. The site and number of hydroxyl groups in the phenol groups are thought to be related to their toxicity to microorganisms with higher antimicrobial capacity observed with higher hydroxylation16. Moreover, some authors have reported that more highly oxidized phenols have higher antimicrobial properties16.
Antimicrobial activity of phenolic products may be due to the destabilization and permeabilization of the cytoplasmatic membrane and enzyme inhibition. Phenols can also inhibit the synthesis of nucleic acids of both gram negative and gram positive bacteria4. In this study, sodium galliate showed a MIC of less than 25mg/ml for all microorganisms tested with the exception of L. monocytogenes and Salmonella spp (50mg/ml). These results agree with a previous report by Borges et al., where gallic acid presented a MIC of 0.5mg/ml for P. aeruginosa and 20mg/ml for L. monocytogenes2. The potassium salts of gallic acid showed MICs of 100mg/ml and higher for all the microorganisms evaluated. In the case of sodium salts of caffeic acid, a low MIC (25mg/ml) was observed for L. mesenteroides; however, a low antimicrobial activity was reported for the other microorganisms evaluated (MIC=100mg/ml). A similar situation was observed for potassium caffeinate. These results contrast with the low MICs obtained for sodium gallic salts. This difference may be due to the fact that gallic acid is a more highly hydroxylated phenol than caffeic acid16.
Sanchez Maldonado et al.18 reported MIC values similar to our study for different compounds including benzoic, caffeic and gallic acids. These authors found that gram positive bacteria are generally more resistant than gram negative microorganisms and that the increase in the number of hydroxyl groups increased the inhibitory activity of hydroxybenzoic and hydroxycinnamic acids. They also stated that the substitution of hydroxyl groups with methoxy groups increased the activity of hydroxybenzoic acids but not of hydroxycinnamic acid.
Interestingly, sodium gallic and caffeic salts presented lower MICs when compared with potassium salts, a fact that contrasts with Borges et al. results2, which establish that potassium chloride has an equivalent antimicrobial effect over some pathogens such as sodium chloride if it is calculated on a molar basis. Moreover, Wang et al.22 have emphasized that the chemical structure and composition of the phenolic products is a determinant of the biological function of the product.
The chemical compounds analyzed in this project show promising results; however, it is important to take into account that MIC results may vary in diverse studies reported in the literature This discrepancy is due to the different methods used for the determination of antimicrobial activity combined with the use of different microorganisms and strains. Furthermore, it is well known that no strain can represent the behavior of a complete species6.
The main objective of this study is to devise chemical products that can reduce pathogens and spoilage microorganisms in foods. The compounds analyzed in this study showed promising results; nevertheless, further studies have to be done to study their safety, solubility, sensory properties (including color and flavor) as well as their stability on different food matrices.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.