The Listeria monocytogenes strains selected in the present study exhibited similar behavior in biofilm formation, independently of the tested conditions (bacteriocin from L. plantarum ST8SH, vancomycin, propolis (a natural antimicrobial product) and EDTA (chelating agent)), individual or in associations. The individual application of vancomycin had better inhibitory activity than that of propolis and EDTA; however, the association of the previously mentioned antimicrobial agents with bacteriocins resulted in better performance. However, when we compared the effects of vancomycin, propolis and EDTA, we could clearly observe that the combined application of bacteriocin and vancomycin was more effective than the combination of bacteriocin and propolis, and bacteriocin and EDTA. Considering the current need to reduce the use of antimicrobials and chemical substances in food processing, propolis can represent an alternative to improve the inhibitory effect of bacteriocins against L. monocytogenes biofilm formation, based on the obtained results. In general, high concentrations of bacteriocin produced by L. plantarum ST8SH were more effective in biofilm inhibition, and similar results were observed for vancomycin and propolis; however, all tested EDTA concentrations had similar effect on biofilm formation.
Las cepas de Listeria monocytogenes seleccionadas en el presente estudio presentaron comportamientos similares en la formación de biofilms, independientemente de los tratamientos a las que fueron sometidas (bacteriocina de Lactobacillus plantarum ST8SH, vancomicina, própolis (produto natural antimicrobiano) y EDTA (agentes quelante)), individual o en combinaciones. La aplicación individual de vancomicina presentó una mejor actividad inhibitoria frente a las aplicaciones individuales de própolis y de EDTA; sin embargo, la combinación de estos agentes antimicrobianos con las bacteriocinas resultó en un mejor desempeño. Se observó claramente que la aplicación combinada de bacteriocina y vancomicina fue más efectiva para controlar el desarrollo de biofilm en comparación con la combinación de la bacteriocina y el própolis o de la combinación de la bacteriocina y el EDTA. Considerando la necesidad actual de reducir el uso de sustancias antimicrobianas y químicas en el procesamiento de alimentos y sobre la base de los resultados obtenidos, se puede afirmar que el própolis representa una alternativa para mejorar el efecto inhibitorio de bacteriocinas contra la formación de biofilm de L. monocytogenes. En general, altas concentraciones de la bacteriocina producida por L. plantarum ST8SH fueron más eficaces en la inhibición del biofilm, y se observaron resultados similares para la vancomicina y el própolis; sin embargo, todas las concentraciones de EDTA evaluadas tuvieron un efecto similar en la formación de biofilm.
Biofilm formation by pathogenic bacteria is considered a serious problem in health care facilities and food industry. Microbial communities develop biofilms for self-protection against unfavorable conditions; this process usually involves more than a single species that exchange information mainly through quorum sensing. Many factors such as the characteristics of the adhering surface, temperature, nutrients, exposition to light, stressing conditions, among others25, can interfere with biofilm formation by microbial communities.
Listeria monocytogenes is a foodborne pathogen known by its ability to form biofilms under different conditions, resulting in economic losses to food industry due to contamination of end products and serious risks for consumers5. Studies on the mechanisms of biofilm formation by different L. monocytogenes strains indicated that the luxS gene plays an important role in this process; this gene is responsible for triggering the quorum sensing response in other microbial species, such as Vibrio harveyi2. L. monocytogenes mutant strains harboring the luxS gene were described as capable of forming dense biofilms23, and many other specific proteins are involved in this process, such as the autoinducer molecule Ai-214. In addition, L. monocytogenes can carry a variety of proteins associated to its pathogenic activity, such as internalins, listeriolysins and phospholipases, which are essential to its virulence cycle9.
The control of L. monocytogenes contamination and biofilm formation is a current challenge for food industries. Sterilization by high temperatures and ultraviolet light, disinfectants and good hygienic practices are standard procedures often used individually or in association to clean potential contamination points in the environment, equipment and utensils. However, there is a current claim by consumers to reduce the use of chemical products in hygienic procedures by the food industries, in order to decrease the chances of residual contamination in end products. Natural products are being increasingly used in cleaning procedures, representing alternatives to avoid biofilm formation in food industries. In this context, natural products such as propolis and antimicrobial proteins produced by lactic acid bacteria (LAB) can be considered alternative strategies to control L. monocytogenes biofilm formation.
Propolis was already described as possessing antibacterial and antifungal properties, due to the presence of active compounds such as galangin (a flavonoid)8 and caffeic acid (a hydroxycinnamic acid)22. Propolis is a resinous mixture collected from honey bees from tree buds, sap flows, or other botanical sources. The biological purpose of propolis has been suggested to be related to different biological contributions, including as a sealant for unwanted open spaces in the hive, and as an antibacterial agent. Since propolis is a natural product, its composition may vary depending on the hive, the geographical area, the season and the plants that are pollinated by bees; however, in general it is composed of plant resins and vegetable balsams, waxes, essential oils, and pollen8,22,24,28. LAB are well known due to their ability to produce bacteriocins, ribosomal synthesized peptides that kill target organisms generally by increasing the permeability of the cytoplasmic membrane, and being used directly as a semi-purified compound, or indirectly via the application of a bacteriocin-producing organism15. Bacteriocin activity can be affected by the presence of chemical surfactants, such as EDTA19, and present a synergetic effect when applied with some antibiotics20,26.
The present study aimed to evaluate alternative procedures to control biofilm formation by L. monocytogenes strains by using the bacteriocins produced by the bacteriocinogenic strain Lactobacillus plantarum ST8SH associated to EDTA, vancomycin and propolis.
Materials and methodsBacterial strains and bacteriocinogenic activityBacteriocin producer strain L. plantarum ST8SH was previously isolated, identified and the produced bacteriocins were characterized27. Pure culture containing bacteriocin producer and other microorganisms used in this study were maintained at −80°C in MRS broth and BHI (BD, Franklin Lakes, NJ, USA) supplemented with 30% (v/v) glycerol.
Bacteriocin-containing cell free supernatant (BCFS) was prepared as recommended in our previous work: L. plantarum ST8SH was grown in MRS broth at 37°C for 24h. BCFS was obtained after centrifugation (10,000×g, 10min, 4°C) and pH was corrected to 6.0 with 1M NaOH, and tested against L. monocytogenes Scott A, L. monocytogenes ATCC 7644, L. monocytogenes 211 and L. monocytogenes 506 to confirm bacteriocin production, according to Todorov et al.27 Bacteriocin activity was recorded as millimeters of inhibition zones, and arbitrary units (AU) per ml27.
Determination of the minimal inhibitory concentration (MIC) of vancomycin and propolisL. monocytogenes (Scott A, ATCC 7644, 211 and 506) were grown in BHI for 24h at 37°C. Cells were washed 2 times with sterile saline solution (0.85% NaCl) and re-suspended in the original volume of trypticase soy broth (TSB, BD). Each L. monocytogenes culture (10μl) was inoculated in 100μl of TSB (BD) on different wells of a flat-bottom 96-well microtiter plate (NUNC, Thermo Scientific). Vancomycin (Sigma–Aldrich, St. Luis, MS, USA) was diluted two-fold in sterile ultrapure water (MilliQ, Millipore, Billerica, MA, USA) and 10μl of each dilution (from 1.0μg/ml to 0.015μg/ml) was added to L. monocytogenes. As positive controls, aliquots of 10μl of each target strain were also transferred to the wells and added with 100μl of TSB (BD) and 10μl of sterile water. The prepared plates were incubated at 37°C for 24h and growth was recorded based on optical density changes (OD) at 550nm on a micro-plate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). In a separate experiment, the effect of commercially available propolis (Apicultura Milliflor, S.P. Ferros, MG, Brazil) as antimicrobial agent against the same L. monocytogenes strains was determined in a similar manner.
Biofilm formationFour L. monocytogenes strains (Scott A, ATCC 7644, 211 and 506) were tested individually to assess their ability to form biofilms, considering the individual and combined interference of BCFS from L. plantarum ST8SH, EDTA, propolis, and vancomycin. Biofilm development was assessed as described by Galvão et al.13 In summary, aliquots of 20μl of a L. monocytogenes culture were transferred to wells of a flat-bottom 96-well microtiter plate, and added with 20μl BCFS from L. plantarum ST8SH (2.25–12800AU/ml), vancomycin (Sigma–Aldrich, 0.015–1.0μg/ml), propolis (Apicultura Milliflor, non-diluted commercial product to 128 times diluted) or EDTA (Sigma–Aldrich, 0.15–10mM). Associations of BCFS from L. plantarum ST8SH with vancomycin, propolis or EDTA were also tested considering the same concentrations. Then, TSB was added in each well to complete the final volume of 140μl. Prepared microtiter plate were incubated at 37°C for 24h. After incubation, culture media were discarded, and the wells of the microtiter plate were washed with phosphate-buffered saline (PBS, pH 7.2) three times to remove non-attached cells. Cells of L. monocytogenes were fixed by the addition of methanol (Sigma–Aldrich), and air dried for 10min. In the next stage, a crystal violet solution (1%, w/v) was added, and after 15min the plates were washed with tap water. After drying, 95% ethanol was added to each well, and the absorbance was measured after incubation for 30min at room temperature (λ=550nm, BioTek Instruments). For each prepared plate appropriate controls were included.
The same approach was considered to check the effect of tested antibacterial substances on previously formed biofilms. Tested L. monocytogenes strains were inoculated individually in microtiter plates, as described above, and added with TSB to complete the final volume of 140μl per well. After biofilm formation (37°C for 24h), cultures were discarded and BCFS from L. plantarum ST8SH, EDTA, propolis, and vancomycin were added individually or in association (as described above), being each well volume completed with sterile water until 140μl, and incubated at 37°C for 150min. Then, the well contents were discarded, when staining and absorbance procedures were conducted as described above. The same blank and positive controls described above were considered in this assay, as well as the interpretation of results.
Detection of virulence markers and luxS geneThe four L. monocytogenes strains were also tested for the presence of virulence and biofilm-related genes. The presence of genes inlA, inlB, inlC, inlJ, plcA, hlyA, actA, and iap was checked by PCR as described by Camargo et al.4 In addition, a PCR protocol described by Bonsaglia et al.2 was used to check the presence of the luxS gene.
ResultsVancomycin exhibited antimicrobial activity against the L. monocytogenes strains considered in this study, and the MIC was determined to be around 0.075μg/ml. Based on these results, vancomycin was considered in concentrations ranging from 1.0 to 0.015μg/ml in the biofilm inhibition assays described above. Moreover, propolis exhibited inhibitory activity against L. monocytogenes target strains even when diluted 16 times from the commercial product used. Based on the obtained results, we selected a range of dilutions (lower and higher them MIC) of propolis in order to explore the anti-Listeria effect of this natural product in combination with bacteriocin produced by L. plantarum ST8SH and to observe possible synergistic effects.
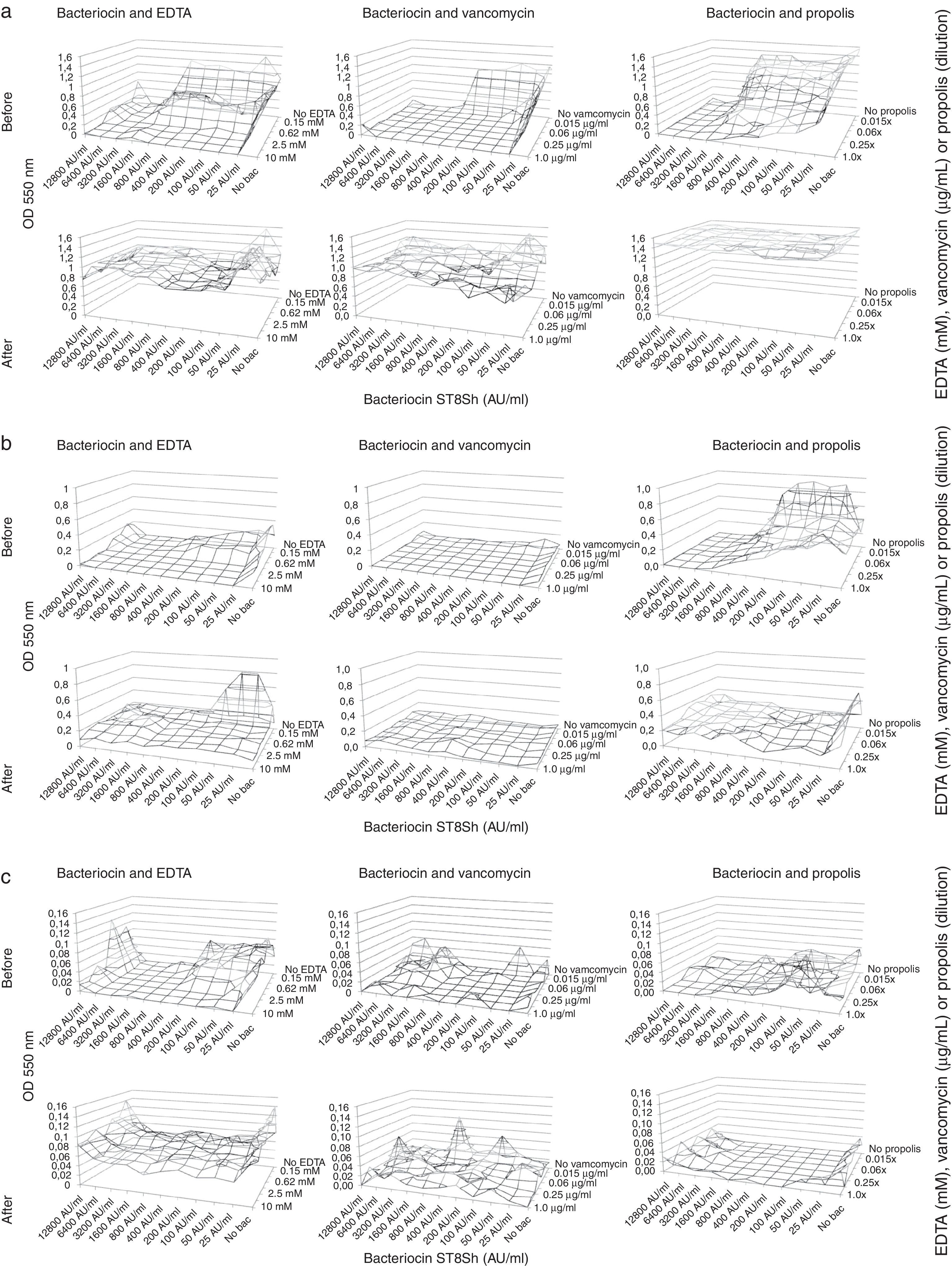
In our study we have explored the combination of bacteriocins produced by L. plantarum ST8SH and vancomycin, propolis and EDTA in order to avoid the formation of biofilms (Fig. 1a–d) and in order to eliminate already formed biofilms (Fig. 1a–d) by four L. monocytogenes strains. The obtained results demonstrated that bacteriocins from L. plantarum ST8SH, vancomycin, propolis and EDTA inhibited biofilm formation by L. monocytogenes (Fig. 1a–d). In addition, the obtained results showed a synergistic effect of bacteriocins and vancomycin, propolis or EDTA (Fig. 1a–d). However, after biofilm formation, the tested substances presented the promising ability to destroy the already formed biofilm structures, despite being tested in isolation or in association (Fig. 1a–d).
(a) Effect of bacteriocin ST8SH (produced by Lactobacillus plantarum ST8SH), EDTA, vancomycin and propolis on biofilm formation (pre- and post-treatment) by Listeria monocytogenes ATCC7644. (b) Effect of bacteriocin ST8SH (produced by Lactobacillus plantarum ST8SH), EDTA, vancomycin and propolis on biofilm formation (pre- and post-treatment) by Listeria monocytogenes ScottA. (c) Effect of bacteriocin ST8SH (produced by Lactobacillus plantarum ST8SH), EDTA, vancomycin and propolis on biofilm formation (pre- and post-treatment) by Listeria monocytogenes 506. (d) Effect of bacteriocin ST8SH (produced by Lactobacillus plantarum ST8SH), EDTA, vancomycin and propolis on biofilm formation (pre- and post-treatment) by Listeria monocytogenes 211.
Nevertheless, when we compared the effects of vancomycin, propolis and EDTA, we could clearly observe that the combined application of bacteriocins and vancomycin was more effective when compared with the combination of bacteriocin and propolis, and bacteriocin and EDTA (Fig. 1a–d). The individual application of vancomycin exhibits better inhibitory activity when compared to individual applications of propolis and EDTA; however, the association of the previously mentioned antimicrobial agents with bacteriocins resulted in better performance (Fig. 1a–d). Moreover, considering the current need to reduce the use of antimicrobial and chemical substances in food processing, propolis can represent an alternative to improve the inhibitory effect of bacteriocins against L. monocytogenes biofilm formation, based on the obtained results (Fig. 1a–d). In general, high concentrations of bacteriocins produced by L. plantarum ST8SH were more effective in inhibiting biofilm formation, and similar results were observed for vancomycin and propolis; however, EDTA concentrations apparently did not influence biofilm formation results (Fig. 1a–d).
Regarding the genetic markers of biofilm formation and virulence, all tested L. monocytogenes strains presented expected amplification products for inlA, inlC, inlJ, hylA, actA, iap, plcA and luxS genes. However, no positive results for amplification of inlB were recorded.
DiscussionAntimicrobial activity of bacteriocins produced by L. plantarum ST8SH against high number of L. monocytogenes strains (including the selected strains in the present study) was previously reported27. LAB bacteriocins with anti-Listeria activity are frequently reported and used to control L. monocytogenes in different food products1,7. It is important to highlight that bacteriocins produced by L. plantarum ST8SH exhibit strong anti-Listeria activity27. In addition, Todorov et al.27 reported that L. plantarum ST8SH carries genes for production of bacteriocins with high similarity to plantaricin 423, a pediocin PA-1 family bacteriocin with possible differences in amino-acids in the C-terminal part of the molecule. Specifically in this strain, the N-terminal of the “pediocin box” is highly conserved, which can explain the wide activity against Listeria spp. This anti-Listeria activity was confirmed in the present study against the target strains of L. monocytogenes (52600AU/ml). Based on these results, BCFS produced by L. plantarum ST8SH were used in concentrations ranging from 12800 to 25AU/ml in the biofilm inhibition assays described above.
Vancomycin is a glycopeptide antibiotic inhibiting the synthesis of the bacterial cell wall. Vancomycin exhibited antimicrobial activity against the L. monocytogenes strains considered in this study, and the MIC was determined to be around 0.075μg/ml. Based on these results, vancomycin was considered in concentrations ranging from 1.0 to 0.015μg/ml in the biofilm inhibition assays described above. L. plantarum has a natural high resistance to vancomycin11, which is due to the presence of d-Ala–d-Lactate in its peptidoglycan rather than in the d-Ala–d-Ala dipeptide. Such resistance is thus intrinsic in most LAB because the antibiotic target is absent and not comparable to the transmissible, plasmid-encoded vancomycin resistance found in enterococcal species10. Although homofermentative lactobacilli are sensitive to vancomycin, many L. plantarum strains possess intrinsic resistance to it, due to the presence of d-alanine–d-alanine ligase-related enzymes11. However, vancomycin is frequently used for control of L. monocytogenes infections, as previously mentioned, vancomycin could be applied in combination with bacteriocinogenic L. plantarum in order to control Listeria spp. contamination. This process can be corrupted by several factors related to the production and expression of bacteriocins, such as availability of nutrients, temperature, water activity, or presence of specific abiotic and biotic factors12.
Propolis exhibited inhibitory activity against L. monocytogenes target strains even when diluted 16 times from the commercial product used. Application of propolis under this concentration can be considered safe, since higher concentrations were recommended for therapeutic use in pharmaceutical preparations. Propolis is a resinous mixture collected from honeybees from tree buds, sap flows, or other botanical sources. The biological purpose of propolis has never been fully understood; however, it is used as a sealant for unwanted open spaces in the hive, and also as an antibacterial agent. Since propolis is a natural product, its composition may vary from hive to hive, and can differ depending on the geographical area, the season and plants that are pollinated by bees28. The analysis of propolis demonstrated the presence of approximately 50 constituents, primarily resins and vegetable balsams (50%), waxes (30%), essential oils (10%), and pollen (5%). Different substances were already described as constituents of propolis, and many of them, such as polyprenylated benzophenones, viscidone, naphthoquinone epoxide, different acids, chrysin, isoflavonoids and phenethyl ester8,22,24 present antimicrobial activity. Propolis also contains persistent lipophilic acaricides, a natural pesticide that deters mite infestations. However, the properties of propolis depend on the exact sources used by each individual hive. Scientific studies have shown that some types of propolis have in vitro antibacterial activity21 and antifungal activity3 due to the presence of active constituents including flavonoids, such as galangin8, and hydroxycinnamic acids, such as caffeic acid22.
In our study we have explored the combination of bacteriocins produced by L. plantarum ST8SH and vancomycin, propolis and EDTA, in order to avoid the formation of biofilms (Fig. 1a–d) and in order to eliminate already formed biofilms (Fig. 1a–d) by four L. monocytogenes strains. Biofilms formed by Listeria spp. strains are usually complex and stable systems, being hardly destroyed by bacteriocins and weak antimicrobial agents4. The obtained results demonstrated that bacteriocins from L. plantarum ST8SH, vancomycin, propolis and EDTA inhibit biofilm formation by L. monocytogenes (Fig. 1a–d). In addition, the obtained results demonstrated a synergistic effect of bacteriocins and vancomycin, propolis and EDTA (Fig. 1a–d). However, after biofilm formation, the tested substances showed a promising ability to destroy the already formed biofilm structures, despite being tested in isolation or in association (Fig. 1a–d). One of the well accepted modes of action of EDTA is related to the disruption of the lipopolysaccharide structure of the bacterial membrane: as a result, the cell membrane becomes more permeable to other antimicrobial agents when an inhibitory synergistic effect can be observed. Usually, EDTA is associated to different antimicrobial substances to improve their inhibitory activity against gram-negative bacteria, such as Pseudomonas aeruginosa17. A synergistic effect of EDTA was also recorded in association with lysozyme, leading to the degradation of the bacterial peptidoglycan layer and resulting in the production of spheroplasts, in which the cell wall has been totally stripped away18. Khan et al.16 investigated the combined application of nisin and EDTA against gram-negative and gram-positive bacteria. Camargo et al.4 demonstrated the synergistic effect of bacteriocins and EDTA for controlling biofilm development by L. monocytogenes strains, using different culture media and growth temperatures.
The L. monocytogenes strains selected in the present study exhibited a similar behavior in biofilm formation, independently of the tested conditions (bacteriocins from L. plantarum ST8SH, vancomycin, propolis and EDTA, as single agents or in associations, Fig. 1a–d). These results were expected, since the selected strains exhibited similar susceptibility to bacteriocins produced L. plantarum ST8SH and vancomycin. However, when we compared the effects of different applied antimicrobial agents, we could clearly observe that the combined application of bacteriocins and vancomycin was more effective when compared with the combination of bacteriocin and propolis, and bacteriocin and EDTA (Fig. 1a–d); however, this was not surprising, since both applied antimicrobial agents (vancomycin and bacteriocin produced by L. plantarum ST8SH) are effective anti-Listeria antimicrobials. Moreover, as an individual antimicrobial agent, vancomycin presents better inhibitory activity when compared to individual applications of propolis or EDTA; however, the association of previously mentioned antimicrobial agents with bacteriocins resulted in better performance (Fig. 1a–d). We will need to consider the current need to reduce the use of antimicrobial and chemical substances in food processing, and in this respect, propolis can represent an alternative to improve the inhibitory effect of bacteriocins against L. monocytogenes biofilm formation, based on the obtained results (Fig. 1a–d). It is important to highlight the fact that high concentrations of bacteriocins produced by L. plantarum ST8SH were more effective on biofilm inhibition, and similar results were observed for vancomycin and propolis; however, EDTA concentrations apparently did not influence the results of biofilm formation (Fig. 1a–d). Chang et al.6 observed that the addition of low EDTA concentrations at the starting time of biofilm formation resulted in a strong biofilm inhibitory effect, but the addition of EDTA after 8h had no inhibitory effect on biofilm formation. Moreover, the safety of using propolis as an alternative for controlling biofilm formation by L. monocytogenes should be addressed, as a single anti-Listeria agent or in combination with bacteriocin substances (Fig. 1a–d); EDTA application can be a good alternative as well; however, restrictions should be considered since it is a chemical healing agent.
Regarding the genetic markers of biofilm formation and virulence, all tested L. monocytogenes strains presented expected amplification products for inlA, inlC, inlJ, hylA, actA, iap, plcA and luxS genes. However, no positive results for amplification of inlB were recorded. Previously, Camargo et al.4 reported the presence of these genes in more than 80 L. monocytogenes isolates obtained from different ecological niches. In another study, Camargo et al.4 reported the highly frequent presence of these genes in different L. monocytogenes strains. These genes play important roles in virulence mechanisms. Some of them comprise Listeria spp. pathogenicity island-1 (LIPI-1) that has been recognized as essential to intracellular colonization, encoding proteins such as LLO, ActA, PlcA, PlcB, Mpl and PrfA. Moreover, other additional genes detected as positive for all isolates, are also essential for full virulence potential9. The control of highly virulent L. monocytogenes strains can be considered a key point in safety issues by the food industry. However, once we observed a similar behavior of strains in biofilm control assays, and similar results of virulence and biofilm genetic markers, no association among biofilm formation and virulence genes could be addressed.
ConclusionsSimilar susceptibility of biofilm formed by investigated L. monocytogenes strains to bacteriocins produced L. plantarum ST8SH and vancomycin (as single antimicrobial agents) were observed and presents better inhibitory activity when compared to individual applications of propolis or EDTA. However, a synergetic effect of combination of bacteriocin and propolis, or bacteriocin and EDTA were recorded. Present results are interesting approach for controlling biofilm formation by L. monocytogenes. Moreover, based on the obtained results, considering the current need to reduce the use of antimicrobial and chemical substances in food processing, propolis can represent an alternative to improve the inhibitory effect of bacteriocins against L. monocytogenes biofilm formation. In addition, it is necessary to address the safety of using propolis as an alternative for controlling biofilm formation by L. monocytogenes, as single anti-Listeria agent or in combination with bacteriocin substances.
Conflict of interestsThe authors declare that they have no conflict of interests.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
This work has been supported by grants from FAPEMIG, CAPES and CNPq.