Egg contamination with microbial pathogens is an enduring worldwide concern. Natural products are frequently recommended as ideal alternatives to substitute synthetic and chemical antimicrobials. Oak galls (Quercus infectoria) are aberrant growths on oak trees that have many medicinal and pharmaceutical applications. Q. infectoria extract (QIE) antimicrobial action was assessed against many microbial species, and used for eggshell decontamination. QIE antimicrobial activity was evidenced against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella Typhimurium and Candida albicans, using different assay methods. Disinfection of eggshell microbial contamination, by immersion in 1% QIE solution, sharply reduced total colony count, yeasts and molds, Enterobacteriaceae. E. coli and S. aureus were completely inhibited after 60min of immersion in QIE. QIE biochemical analysis revealed elevated contents of phenolic and flavonoid compounds. The captured micrographs of S. aureus cells treated with QIE showed strong alterations in cell morphology; cells were entirely lysed and ruptured after 6h of treatment. QIE can be recommended as an effective and natural disinfectant for decontaminating eggshells from pathogenic microorganisms.
La contaminación de huevos con patógenos microbianos es un problema constante en todo el mundo. Con frecuencia se recomiendan diversos productos naturales como alternativas ideales para sustituir a los antimicrobianos sintéticos. Las agallas de roble (Quercus infectoria) son de crecimiento aberrante en los robles y tienen muchas aplicaciones medicinales y farmacéuticas. Se evaluó la acción antimicrobiana del extracto de Quercus infectoria (QIE) contra varias especies microbianas y también este se aplicó para la descontaminación de cáscaras de huevo. La actividad antimicrobiana del extracto de QIE se evidenció en relación con Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium y Candida albicans, utilizando diferentes métodos de ensayo. La inmersión de las cáscaras de huevo en extracto de QIE al 1% logró una fuerte reducción del recuento total de colonias, de levaduras y de mohos, y de miembros de Enterobacteriaceae. La inmersión durante 60min inhibió completamente el desarrollo de E. coli y S. aureus. El análisis bioquímico del extracto de QIE reveló que este tiene un contenido elevado de compuestos fenólicos y de flavonoides. Se documentó mediante micrografías la presencia de grandes alteraciones en la morfología celular de S. aureus tras la exposición al extracto de QIE: las células se lisaron completamente y se rompieron después de 6h de tratamiento. El extracto de QIE se puede recomendar como un desinfectante eficaz y natural para descontaminar cáscaras de huevos de microorganismos patógenos.
Food preservation is a challenging issue faced by scientists, industry overseers, health observers and regular customers. Food could be decontaminated and preserved using specific compounds, e.g. preservatives or antimicrobials, which are defined as the added compounds that have the ability to kill or hinder microbial growth in foods6.
Microbial contamination of eggs with pathogenic microorganisms is a serious health concern worldwide. Eggshells may be contaminated with microorganisms at different industrial stages, e.g. production, processing, preparation and packaging. Contamination and microbial transmission may be “vertical”, during egg formation in the ovaries, or “horizontal”, through egg exposure to the surrounded contaminated environment8. The correlation between the average of eggshell contamination and penetrating microbial pathogens into the egg contents was reported in many previous investigations9,23.
Although there are many approved chemical food disinfectants and preservatives from international regulatory agencies, to be applied as food antimicrobials, there is a strong need for finding more effective agents originated from natural sources30,31,35. The principal advantages of the application of natural disinfectants and antimicrobials are their biodegradability, high biosafety level, wide spectrum and non-accumulating properties6,7,33.
Many reports provided strong indications that the extracts of medicinal plant could be ideal sources for producing new antimicrobial compounds, especially against antibiotic-resistant strains5,11,29.
Oak galls (Quercus infectoria) are abnormal round-shaped growths, which may appear on young oak tree branches27. Gall powder and its extracts were traditionally used to treat many disorders, diseases and symptoms, including menorrhagia, dysentery, diarrhea, internal hemorrhages, gonorrhea, tonsillitis, and impetigo3. Pharmacologically, it was suggested that galls have potent bioactivities, e.g. antioxidant, antibacterial, antifungal, antiviral, larvicidal, antiamoebic, antidiabetic, anti-inflammatory, antivenin, and wound healing18,25,32. Gall applications, in food-related sectors, were also reported after being washed with running water to exclude bitter tannins before cooking. After that, galls could be powdered and applied as thickening agents in stews, or used as cereal supplements for making bread; nutgall powder and extract can be also used as a coffee substitute, e.g. as tea or herbal drink for health promotion18.
Therefore, the current study was designed to evaluate Q. infectoria extract as a potential antimicrobial agent for decontaminating eggshells and to analyze its possible antimicrobial mode of action.
Materials and methodsOak gall extract preparationOak galls, Q. infectoria Olivier (Fagaceae), were obtained from the Medicinal and Aromatic Plants Research Department, Agricultural Research Center, Giza, Egypt. Galls were dried with hot air at 45°C for 24h, then dried materials were powdered using an electrical grinder and the powder was sieved to get ∼60mesh particle size. Two hundred grams of gall powder were immersed in 1l of 70% ethanol and agitated at 230×g for 6h, using a rotary shaker. QIE was filtered in a Buchner funnel through filter papers, Whatman No. 41, to eliminate the plant particles, which were re-extracted with 500ml of the solvent and filtered and the total extracts were combined and subjected to flash evaporation at reduced pressure (Büchi, Flavil, Switzerland) at 40°C to discard about 90% of solvent till constant weight was attained. The final dry extract was further dried under vacuum in a desiccator, weighed and powdered. QIE powder was then suspended in distilled water, by vigorous agitation at 45°C, to reach a final concentration of 10% (w/v). Finally, extract solution was sterilized using a syringe filter (0.22μm pore size, sterilized) and kept at 4°C, in sterile dark bottles.
Microbial strainsDifferent microbial strains were used for examining the antimicrobial activity of QIE: Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 25006, Salmonella Typhimurium ATCC 23852, and Candida albicans ATCC 10231. The bacterial strains were propagated at 37°C in nutrient broth (NB) or nutrient agar (NA) under aerobic conditions, whereas C. albicans was grown in yeast malt broth (YMB) and maintained on yeast malt agar (YMA). All microbial media were purchased from Difco Lab., Detroit, MI.
Determination of extract antimicrobial activityThe antimicrobial activity of QIE was qualitatively and quantitatively assessed, by two different determinations: zone of growth inhibition (ZOI), using the disc diffusion assay, and the determination of minimal inhibitory concentrations (MIC) (according to Tayel et al.34), using the suitable growth media for each microbial strain. The reduction in microbial counts was evaluated after their treatment with each relevant MIC, after 1h and 5h of the exposure, using the standard plate count method for comparing.
Experimental infection of eggsOne hundred and twenty five chicken eggs (grade A, 3–24h old) were obtained from the poultry farm – Kafrelsheikh University. Eggs were firstly washed then sterilized through immersion in 1% sodium hypochlorite solution for 30min, rewashed with sterilized water and allowed to dry in aseptic bags. Chicken fecal samples (80 samples weighed ∼1000g) were collected from farm litter, aseptically transported to the laboratory and suspended in 4l of buffered peptone water (BPW), well homogenized and filtered through cheesecloth. The filtrate was used to simulate natural egg contamination with fecal microorganisms.
For the experimental contamination, eggs were immersed in fecal suspension for 60min, in plastic bags, and then allowed to dry in a laminar flow chamber for 2h14.
QIE application for egg disinfectionA stock solution of 1% QIE in sterilized water was prepared. Contaminated eggs (previously immersed in the fecal suspension) were dipped in the disinfection solution for 15, 30 and 60min, then subjected to microbiological analysis. Decontaminated and control eggs (uncontaminated) were individually immersed in sterile BPW (50ml/each egg), well shaken, serially diluted and 0.1ml from the BPW dilutions were spread on appropriate agar media (See below) to count the viable colonies after incubation for each examined microbial group.
Each group for microbial examination contained five eggs and their mean results were calculated.
Microbiological examinationsDifferent microbial analyses were conducted according to the reference test methods of the British Standards Institution (BSI) to evaluate the effectiveness of QIE as a natural antimicrobial and disinfection agent for chicken eggs, as follows:
- -
Enumeration of total aerobic microorganisms, according to ISO 4833:2003.
- -
Enumeration of molds and yeasts, according to ISO 21527-1:2008.
- -
Enumeration of E. coli, according to ISO 16649-2:2001.
- -
Detection and enumeration of Enterobacteriaceae, according to ISO 21528-2:2004.
- -
Enumeration of coagulase positive staphylococci and S. aureus, according to ISO 6888-1:1999.
The phytochemical analysis of QIE was conducted in the Food Chemistry Lab., Food Technology Research Institute, National Research Center, Giza, Egypt. The quantification of phenolic contents in QIE was carried out according to the method illustrated by Spigno et al.28, whereas flavonoid contents were determined according to Mattila et al.21, using an HPLC system coupled with diode array detection (HPLC-DAD) (Hewlett-Packard, Series-1050, Palo Alto, CA).
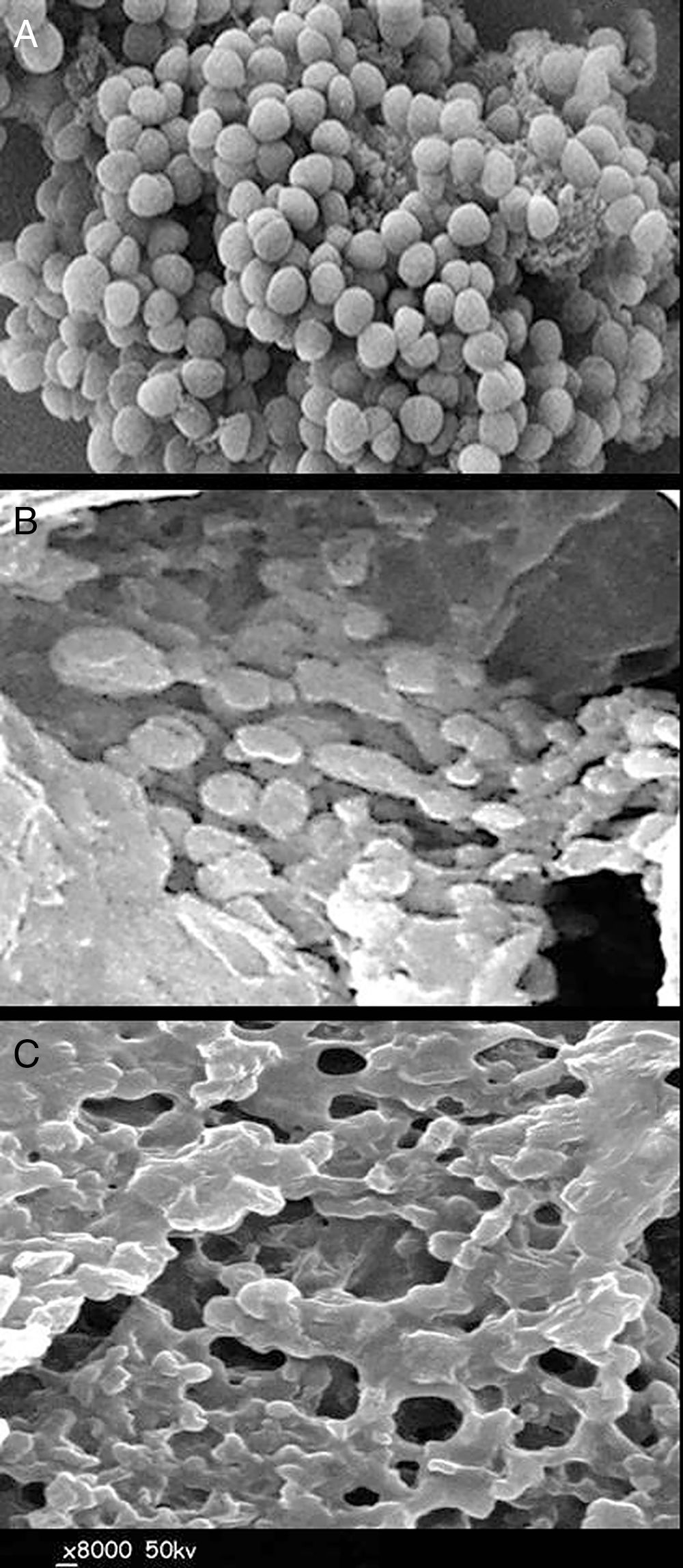
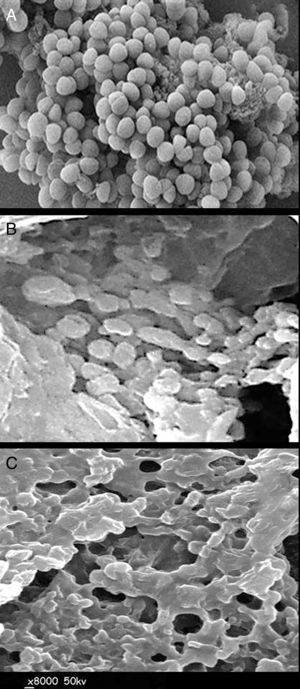
Scanning electron microscopy imagingThe micrographs illustrating morphological alterations in S. aureus bacterial cells were captured using scanning electron microscopy imaging (SEM; Hitachi S-500, Tokyo, Japan) after treatment with QIE20. The bacterial cells were firstly fixed using a primary fixative solution containing 2% paraformaldehyde in 0.1M Na-Cacodylate buffer and 2.5% glutaraldehyde at pH 7.3 for 30min. The fixed samples were repeatedly rinsed with ultrapure water, and were then dehydrated using a series of ethanol concentrations (10, 30, 50, 70, 90 and 100%). The critical point dryer (Tousimis, Rockville, MD, USA) was then used for immediate drying of the dehydrated samples, then they were sputter-coated with gold/palladium after mounting onto SEM stubs. The electron micrographs were captured, at 50kV and 8000×, for the control samples and after 3 and 6h from the exposure to QIE; the captured sections were chosen depending on the morphological alteration in treated bacterial cells.
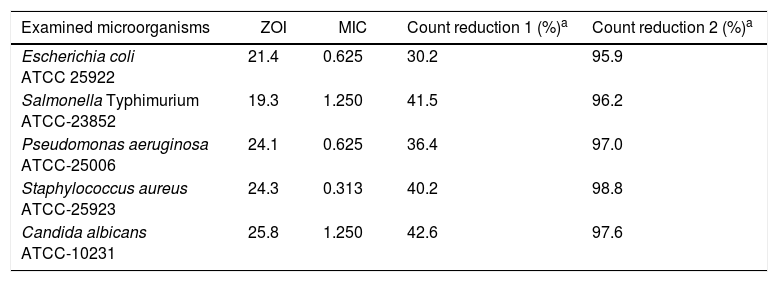
ResultsThe antimicrobial activity of QIE against the examined microbial strains is shown in Table 1 and was evidenced against the entire species, using both the quantitative (MIC) and qualitative (ZOI) assays. It could be claimed that the most sensitive strain to QIE activity was S. aureus, whereas the most resistant one was S. Typhimurium. With regard to microbial count reduction, after exposure to the corresponding MICs from QIE, it was recorded that the microbial viability decreased to 57.4–69.8%, after 1h from exposure, and to less than 4.1%, after QIE exposure for 5h. Only 1.2% from exposed S. aureus cells could survive after 5h of treatment with QIE.
Antimicrobial activity of Quercus infectoria extract measured qualitatively as zone of inhibition diameter (ZOI, mm) and quantitatively as minimal inhibitory concentrations (MIC, mg/ml).
| Examined microorganisms | ZOI | MIC | Count reduction 1 (%)a | Count reduction 2 (%)a |
|---|---|---|---|---|
| Escherichia coli ATCC 25922 | 21.4 | 0.625 | 30.2 | 95.9 |
| Salmonella Typhimurium ATCC-23852 | 19.3 | 1.250 | 41.5 | 96.2 |
| Pseudomonas aeruginosa ATCC-25006 | 24.1 | 0.625 | 36.4 | 97.0 |
| Staphylococcus aureus ATCC-25923 | 24.3 | 0.313 | 40.2 | 98.8 |
| Candida albicans ATCC-10231 | 25.8 | 1.250 | 42.6 | 97.6 |
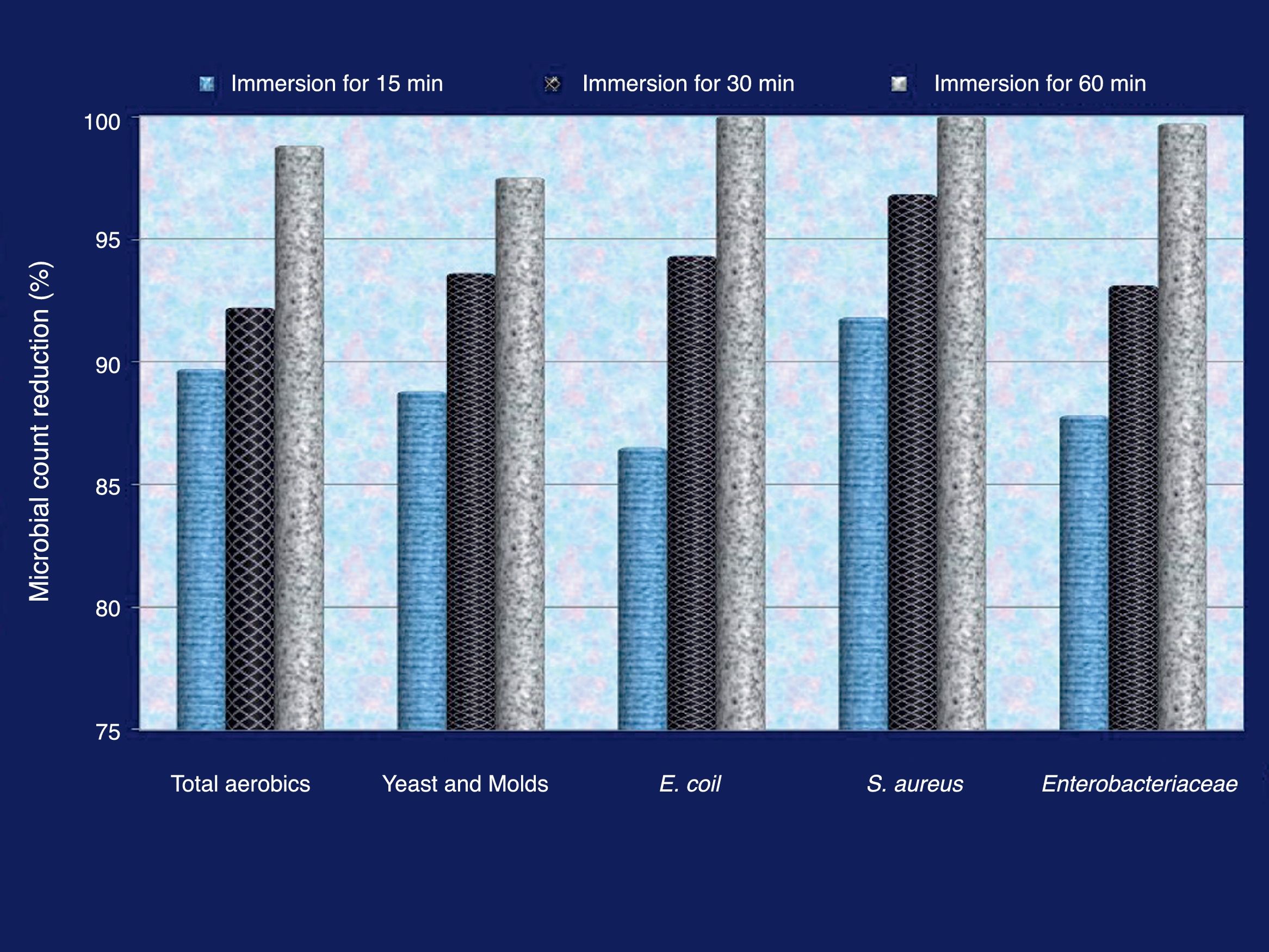
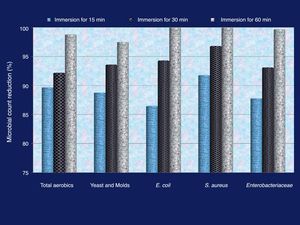
The consequence of eggshell decontamination through immersion in 1% QIE solution on the count of contaminating microorganisms is shown in Figure 1. The number of all examined microbial groups severely decreased after immersion in QIE and the count decrement continued with the prolongation of the immersion period. S. aureus was the most sensitive group to the sterilization action of QIE. Both E. coli and S. aureus were entirely inhibited after 60min of immersion in QIE solution, whereas the remaining percentage of viable cells were 1.2, 2.5 and 0.3% for the total aerobic colony count, yeast & molds and Enterobacteriaceae groups, respectively, after the same immersion time.
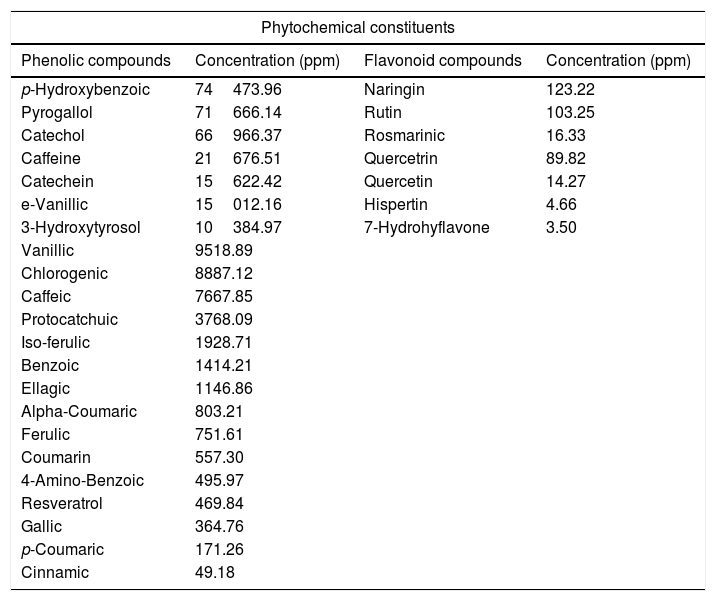
The biochemical analysis of QIE content from phytochemical constituents (Table 2) revealed that the extract was very rich in its contents of phenolic compounds. The main phenolic compound in the QIE was p-hydroxybenzoic acid (PHBA), with a concentration of 7.45% followed by pyrogallol, catechol, caffeine, catechein, e-vanillic acid and 3-hydroxytyrosol with percentages of 7.17, 6.70, 2.17, 1.56, 1.50 and 1.04%, respectively. On the other hand, the lowest concentrations of QIE phenolic constituents were recorded for cinnamic, p-Coumaric, gallic acids and resveratrol, respectively. The main flavonoid compounds in QIE were naringin and rutin with a concentration of 123.2 and 103.3ppm, whereas the lowest concentrations of flavonoid compounds were for 7-hydrohyflavone and hispertin, with 3.5 and 4.7ppm, respectively.
Biochemical analysis of phytochemical constituents in Quercus infectoria extract.
| Phytochemical constituents | |||
|---|---|---|---|
| Phenolic compounds | Concentration (ppm) | Flavonoid compounds | Concentration (ppm) |
| p-Hydroxybenzoic | 74473.96 | Naringin | 123.22 |
| Pyrogallol | 71666.14 | Rutin | 103.25 |
| Catechol | 66966.37 | Rosmarinic | 16.33 |
| Caffeine | 21676.51 | Quercetrin | 89.82 |
| Catechein | 15622.42 | Quercetin | 14.27 |
| e-Vanillic | 15012.16 | Hispertin | 4.66 |
| 3-Hydroxytyrosol | 10384.97 | 7-Hydrohyflavone | 3.50 |
| Vanillic | 9518.89 | ||
| Chlorogenic | 8887.12 | ||
| Caffeic | 7667.85 | ||
| Protocatchuic | 3768.09 | ||
| Iso-ferulic | 1928.71 | ||
| Benzoic | 1414.21 | ||
| Ellagic | 1146.86 | ||
| Alpha-Coumaric | 803.21 | ||
| Ferulic | 751.61 | ||
| Coumarin | 557.30 | ||
| 4-Amino-Benzoic | 495.97 | ||
| Resveratrol | 469.84 | ||
| Gallic | 364.76 | ||
| p-Coumaric | 171.26 | ||
| Cinnamic | 49.18 | ||
The consequence of QIE exposure on the morphology and viability of S. aureus cells is shown in Figure 2. The captured micrographs of untreated (control) bacterial cells showed that they had a normal, unified and smooth structure (Fig. 2A). After 3h of exposure to QIE, the effect was remarkably strong on cell morphology (Fig. 2B); most cells were lysed and their interior contents released, the remaining intact cells had enlarged and buffy walls with notable initiation of lysis. Upon completion of the QIE exposure period (after 6h), all S. aureus cells became completely lysed and ruptured; the only observable materials were cell wall residues and the interior cell components released (Fig. 2C).
DiscussionPlants ordinarily protect themselves from invaders and microorganisms via the production of secondary metabolites, which generally represent miscellaneous arrays derived from alkaloid, phenylpropanoid, isoprenoid, and fatty acid/polyketide pathways15; therefore, these are the main reasons for screening plants as potential sources for antimicrobial agents10. The antibacterial power of QIE was strong against Gram positive bacteria such as S. aureus compared to its action against treated Gram negative bacterial strains; a fact that was confirmed by ZOI diameters and the required MIC values. Accordant results were previously reported from other studies, which indicated that Gram positive bacteria are generally more susceptible to be inhibited by plant extracts than Gram negative strains5. The alteration in bacterial cell wall composition could explain the variation in microbial sensitivity to QIE or other plant extracts11.
The applied solvent in this study for the extraction of bioactive compounds in Q. infectoria, contained 70% ethanol and 30% water. It was reported that the usage of alcoholic solvents is commonly recommended for the extraction of phenolics from natural origins because they can yield higher amounts of total extract compared with other types of solvents28.
As tannins are the major compounds in QIE, which is soluble in water, the used solvent had a portion of water to dissolve the high amounts of total tannin contents1.
Medicinal plants have been recurrently applied in many nutritional, pharmaceutical, medicinal and health promoting fields; this ethnopharmacological usage confidently warrants their compatibility and biosafety for man5. Many traditional and modern food applications were reported for Q. infectoria powders and extracts18, which could indicate the potential biosafety of nutgalls for human uses.
The application of QIE for disinfection of eggshells exhibited powerful antimicrobial activity against contaminating microbial groups; which could be correlated with the high content of QIE from bioactive compounds12. It was affirmed that many flavonoids and phenolics, which are contained with high percentages in QIE, have a potent antimicrobial and antioxidant activities13,26; the combination of these functional compounds is supposed to strengthen their action.
Oak galls arise because of tree attacks by insects, thus they contain many defence phytochemicals. This characteristic could explain the wide variety of bioactive compounds found in Q. infectoria extract. It was reported that intact plants might include many bioactive compounds, e.g. glycosides, flavonols, alkaloids, flavones, lactones, organic acids, phenolic compounds and protein-like compounds, whereas other antimicrobial compounds, e.g. isothiocyanates phytoalexins, phenolic compounds and sulfoxides may be found in post-infection plants19.
The highest concentration of phenolic compounds in QIE was reported for p-hydroxybenzoic acid (74474ppm), which was reported to have a powerful antimicrobial activity against many microbial strains, and this was also reported for vanillic, caffeic and ferulic acids22.
From the detected phenolic compounds with high concentration in QIE, pyrogallol and catechol (the allelochemicals that belong to plant-synthesized phenolic compounds) exhibited concentrations of 71666 and 66966ppm, respectively. The antimicrobial activities of both pyrogallol and catechol were confirmed against many bacterial and fungal strains17.
Phenolic allelochemicals are assumed to serve as defensive agents against microbial phytopathogens and to act as signal molecules in the interactions between plants with pathogens16.
With regard to flavonoid content in QIE, it was reported that many flavonoid compounds, e.g. rosmarinic acid, could have antimicrobial potential against a wide variety of microorganisms4.
Tannin content in QIE is typically high and contains both the hydrolysable and condensed types33. Both tannin types were used for the treatment of many diseases, especially the hydrolysable tannins, which were more medicinally applied as antifungal and antibacterial agents15; this could be a further explanation for the antimicrobial activity of QIE24.
S. aureus was selected as a model organism to elucidate the antimicrobial action of QIE using scanning electron microscopy, because it was recorded in the study as the most sensitive strain to the extract, using the different antibacterial assays. Thus, it could be expected to exhibit various explanations for QIE modes of action, through SEM imaging.
With regard to the captured micrographs of S. aureus cells treated with QIE (Fig. 2), it could be assumed that some potential bioactive compound(s) in QIE may have a metabolic interference in bacterial growth, development or function.
From the alteration in bacterial morphology after treatment with QIE for an extended duration, it could be suggested that the extract has a time-dependent killing action. The QIE mechanism of action could also be thought to depend on the degradation of bacterial cell walls, destruction of cytoplasmic membrane proteins, leakage of cell contents, coagulation of cytoplasm, reduction in the proton motive force or binding with some synthesis proteins1,12.
It was suggested that extracts of medicinal plants with high tannin content, e.g. QIE, target the enzymes involved in cell wall synthesis of resistant S. aureus strains2.
The current study could serve as a starting point for further investigations concerning the antimicrobial action, application and biosafety of individual/combined purified compounds in QIE.
From the results achieved in this study, it can be concluded that oak gall (Q. infectoria) extract has antimicrobial activity, which can be applied as a natural disinfectant to protect chicken eggs from microbial contamination.
FundingThere are no funding sources to declare.
Conflict of interestThere are not any conflicts of interest.