The aim of this study was to evaluate different production methodologies of probiotic macrocapsules with high bacterial densities destined to lactating calves. Three types of capsules containing Lactobacillus casei DSPV318T and Lactobacillus plantarum DSPV354T were prepared from an overnight culture in whey medium: (1) mixing the culture with calcium alginate and then, reincubating the capsules in whey (RC); (2) concentrating the biomass by centrifugation and mixing the pellet with calcium alginate (CC) at different concentrations with respect to the initial culture (5X and 12.5X); (3) CC with cryoprotectants: whey permeate (Per) and glycerol (Gly). Chitosan coating was evaluated. Capsules were freeze-dried and viability was assessed before freezing, after freeze-drying and every two weeks for 84 days of storage at room temperature, 4°C and −20°C. CC showed higher cell densities than RC. Storage temperature affected viability: greater viability at lower temperature. Moreover, the effect of temperature was influenced by other factors, such as capsule coating, culture neutralization and cryoprotectants. Coating improved viability at room temperature; however no effect was observed at 4°C. Culture neutralization allowed greater survival during storage. Cryoprotectants improved viability during freezing, but they also generated a positive or negative effect depending on storage temperature. The best results were: at refrigeration Gly12.5X exhibited counts above 109CFU/capsule until day 70 and Per12.5X until day 56 of storage and at −20°C Gly12.5X showed counts above 109CFU/capsule until the end of the study (84 days). A 109CFU capsule is the daily dose per calf which would facilitate the administration of this probiotic inoculum to field animals.
El objetivo de este estudio fue evaluar diferentes metodologías de producción de macrocápsulas probióticas con altas densidades bacterianas, destinadas a terneros lactantes. Se prepararon cápsulas con Lactobacillus casei DSPV318T y L. plantarum DSPV354T a partir de cultivos overnight en suero de queso, de 3 maneras: 1) mezclando el cultivo con alginato de calcio y luego reincubando las cápsulas en suero (RC); 2) concentrando la biomasa por centrifugación y mezclando el sedimento con alginato de calcio (CC) en diferentes concentraciones con respecto al cultivo inicial (5X y 12,5X), y 3) CC con crioprotectores: permeado de suero (Per) o glicerol (Gly). Se evaluó el recubrimiento con quitosano. Las cápsulas se liofilizaron y se evaluó la viabilidad antes de la congelación, después de la liofilización y cada 2 semanas durante 84 días de almacenamiento a temperatura ambiente a 4 o a −20°C. Con el sistema CC se alcanzaron mayores densidades celulares que con el RC. La temperatura de almacenamiento afectó la viabilidad: se observó una mayor viabilidad a menor temperatura. Además, otros factores incidieron en el efecto de la temperatura: el recubrimiento de las cápsulas, la neutralización del cultivo y la adición de crioprotectores. El recubrimiento mejoró la viabilidad a temperatura ambiente, pero no se observó ningún efecto a 4°C. La neutralización del cultivo permitió una mayor supervivencia durante el almacenamiento. Los crioprotectores mejoraron la viabilidad durante la congelación, pero también generaron un efecto positivo o negativo dependiendo de la temperatura de almacenamiento. Con el almacenamiento en refrigeración se obtuvieron recuentos por encima de 109 UFC/cápsula hasta el día 70 y con Gly12,5X y hasta el día 56 con Per12,5X. Con el almacenamiento a −20°C se obtuvieron cápsulas Gly12,5X con más de 109 UFC/cápsula hasta el final del estudio (84 días). Una cápsula de 109 UFC es la dosis diaria por ternero que facilitaría la administración de este inóculo probiótico a los animales en el campo.
The inclusion of antibiotics in the commercial diets of calves is a widespread practice which reduces the occurrence of pathogens28. It improves feed conversion, weight gain and reduces diarrheal diseases in calves4,32. However, this practice has been placed under scrutiny. On the one hand, many of the antibiotics used in animal production systems are also used in human medicine. On the other hand, the indiscriminate use of antibiotics leads to the development of resistant-bacterial populations. This situation hinders antibiotic therapy, both in animals and humans, causing several economic losses2 and important problems in Public Health4. In Argentina, the Servicio Nacional de Sanidad y Calidad Agroalimentaria (National Agri-food Health and Quality Service)30 will ban the use of antibiotics as growth promoters as from the year 2019. Therefore, it is necessary to search alternative tools that can provide a solution to this problem.
Probiotics are defined as “live microorganisms that are administered in adequate number conferring a health benefit to the host”16. Authors have reported that probiotic supplementation modifies intestinal microbiota34, reduces diarrhea incidence37, decreases mortality rate18, improves performance parameters4,17, and may be an alternative to the use of antibiotics as growth promoters in young calves32.
The administration of probiotics in the productive systems requires high loads of living microorganisms to reach the sites of action, occupy a place within the large intestinal ecosystem and finally exert their probiotic effect. This requirement raises the need to find a carrier capable of containing high amounts of probiotics and maintaining its viability throughout the shelf-life of the product. Macrocapsules, designed to simulate the size and shape of pelleted grain of the starter feed for calves33, are a suitable carrier for transporting probiotic inocula to the farm and administering them to the animals by mixing with feed. Calcium alginate has been frequently used for the encapsulation and immobilization of LAB particularly due to its simplicity of handling, non-toxicity, low cost, and acceptance as a food additive10. However, Ca-alginate capsules are chemically unstable in contact with various cationic chelating agents such as phosphate, citrate and lactate which can cause the disruption or dissolution of the capsule26. Some authors improved the viability of encapsulated microorganisms through the formulation of semipermeable layers of chitosan around the alginate capsules. Thus, the effects of calcium chelating agents are reduced. In addition, the structure of the capsule denser and stronger, avoiding rupture and release of cell contents1.
Although it is agreed that probiotic products must contain a suggested minimum level (SML) of 106CFU of viable cells/g to assure sufficient bioavailable bacteria and exert a functional effect39, the daily probiotic dose that calves have to consume should be at least 109CFU35. Therefore, the higher bacterial concentration per capsule, the lower number of capsules to be given to the calves. In this way, the implementation of methodologies that increase cell densities at low cost, and are able of providing protection to the strains against the above mentioned factors is very important when produced at industrial scale.
The bacterial load of the macrocapsules will depend on several factors, such as proliferation capacity of the strains in culture media, capsule production method, materials included in the matrix conformation, moisture and storage conditions. Bacterial proliferation requires appropriate culture media to provide the nutrients required by the microorganism for its development. Industrial products such as cheese whey has been used as a culture medium for the propagation of probiotic inocula6,25. The use of whey would not only reduce biomass production costs, but may have other positive effects at other stages of production. The whey proteins present in the culture medium could form part of the capsule, which would bring additional advantages, such as the decrease in the amount of the polymerizing agent that must be added for the formation of the capsule. It is also known that cheese whey proteins40,41 can exert a cryoprotective effect, improving the viability of the bacteria during the lyophilization process and during freezing storage, avoiding a decrease of microbiological counts over the shelf life of the product. In this sense, the production of probiotic macrocapsules with high cell densities would generate lower production costs in materials to form the container matrix, since each dose would be concentrated in one single capsule. In turn, this would simplify administration on the farm, giving one single capsule per calf per day.
Primary milk production requires the development of new feeding strategies to improve performance and ensure animal health, macroencapsulation of probiotics is presented as an interesting tool to be applied during artificial calf rearing. The aim of this work was to evaluate different methodologies for the production of probiotic macrocapsules with high cellular densities destined to lactating calves.
Materials and methodsBacterial strainsTwo strains of bovine origin with in vitro and in vivo probiotic properties17,34 were used: Lactobacillus casei DSPV 318T (GenBank accession number: FJ787305) and Lactobacillus plantarum DSPV 354T (GenBank accession number: FJ751793).
Culture conditionsBoth strains were activated on MRS agar (de Man, Rogosa, Sharpe) at 37°C for 72h in anaerobiosis. Subsequently, two consecutive cultures were performed in MRS broth at 37°C for 16h. Then, strains were co-culture in cheese whey (70g/l), at 37°C for 24h, until a concentration of 109CFU/ml.
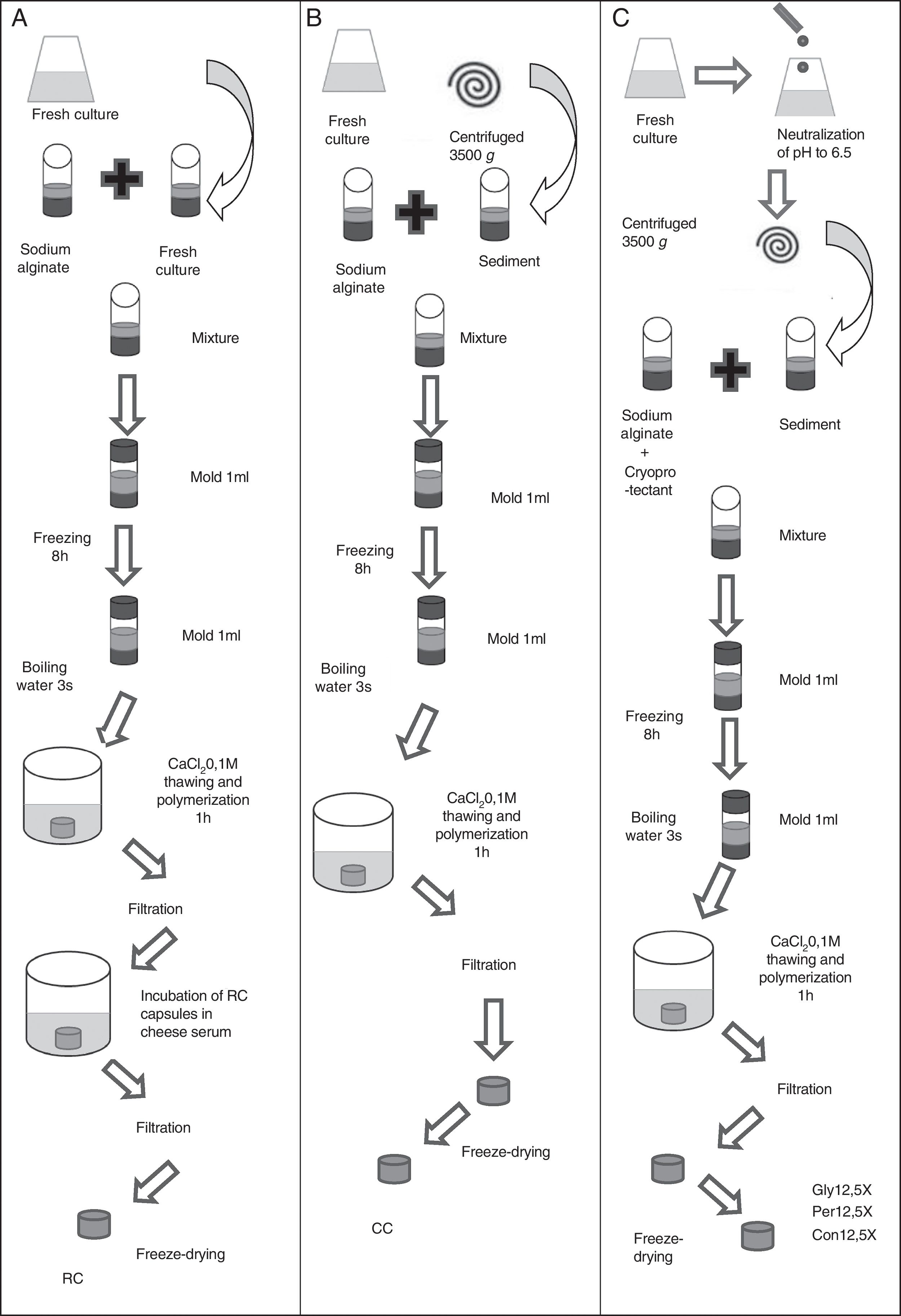
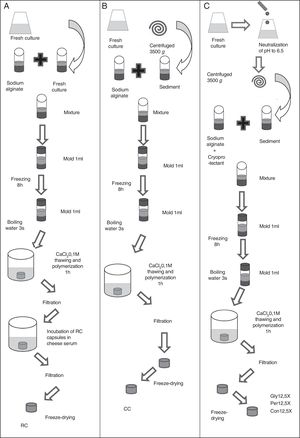
Production methods of macrocapsulesTwo types of macrocapsule production methods were used. Reincubated capsules (RC) was the first type, according to the methodology described by Soto et al.33 After the incubation time of the culture in cheese whey, sodium alginate (40g/l) was added in a 1:1 (v/v) ratio and this mixture was poured in 1ml molds. These cultures reached final alginate concentrations of 20g/l7. The molds were stored at −20°C for 8h to solidify the mixture. They were submerged in boiling water for three seconds to remove the capsules from the mold, and then they were submerged in a solution of 0.1M CaCl2 for 1h to allow alginate polymerization. Finally, the capsules were recovered by filtration and incubated in cheese whey (70g/l) for 12h to increase the biomass inside the capsules (Fig. 1A).
Schematic representation of macrocapsule production from fresh culture to preparation of dried macrocapsules. (A) Reincubated capsules (RC). (B) Concentrated capsules (CC). (C) Capsules with cryoprotectant (Gly12.5X: concentrated capsules 12.5X with glycine; Per12.5X: concentrated capsules 12.5X with whey permeate; Con12.5X: control capsules 12.5X without cryoprotectants).
The other type of capsule was made by concentrating the biomass before capsule production (CC). Overnight cultures were centrifuged at 4500×g for 10min. Pellets were suspended in cheese whey at a concentration of 5X (CC5X) and 12.5X (CC12.5X) with respect to the initial volume of the cultures. Sodium alginate (40g/l) was added in a 1:1 (v/v) ratio to both cultures to reach final alginate concentrations of 20g/l. The capsules were kept at −20°C for 8h to solidify their contents. They were submerged in boiling water for 3s to remove the capsules from the mold and then, they were submerged in a solution of 0.1M CaCl2 for 1h to allow alginate polymerization (Fig. 1B).
Coating and storage of capsulesHalf of the RC, CC5X and CC12.5X were immersed in a chitosan solution for 40min to achieve a coating. The chitosan solution was prepared at a concentration of 4g/l in doubly distilled water acidified with glacial acetic acid (4.4ml/l). The pH was adjusted to 5.8±0.2 with NaOH and the solution was sterilized for 15min at 121°C26.
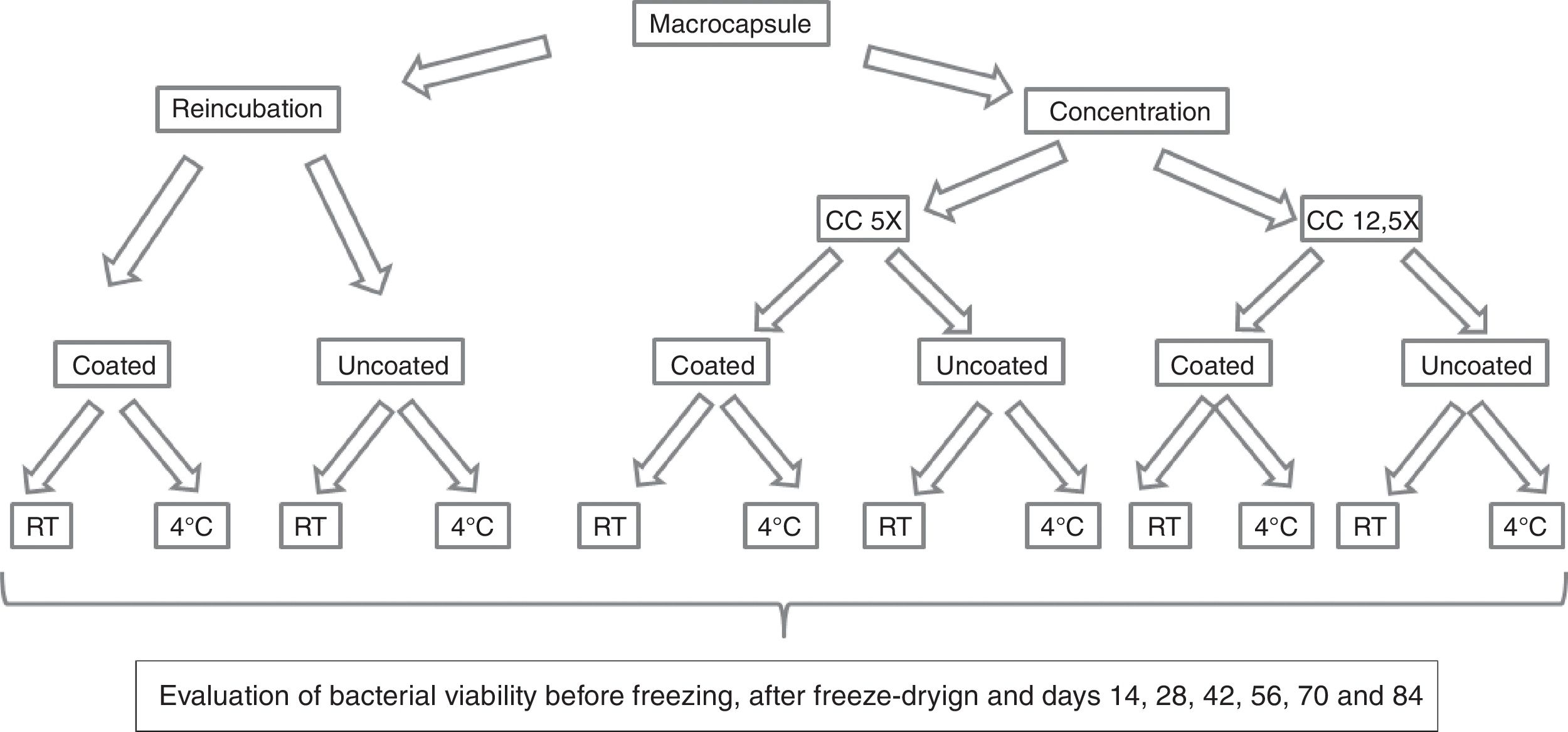
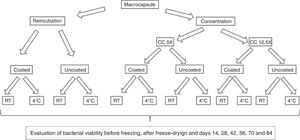
All capsules (coated and uncoated RC, CC5X and CC12.5X) were placed at −80°C for 8h. Freeze-drying was carried out at 0.044mbar for 12h at −54°C (Martin Christ ALPHA 1–4 LD plus Osterode am Harz, Germany). Half of the coated RC, CC5X and CC12.5X were stored at 4°C (T4), while the other half were stored at room temperature (RT), at approximately 25°C. The same procedure was employed for uncoated RC, CC5X and CC12.5X (Fig. 2). The experiments were performed in triplicate.
Experimental design for the production and conservation of the capsules in different temperature conditions. RC: reincubated capsules; CC: concentrated capsules. CC5X: concentrated capsules 5X with respect to the initial volume culture; CC12.5X: concentrated capsules 12.5X with respect to the initial volume culture; RT: storage of capsules at room temperature; 4°C: storage of capsules under refrigeration conditions.
In order to improve bacterial viability during storage, after the fermentation period, the pH of the medium was brought to 6.5 by the addition of 6M NaOH. Neutralized cultures were centrifuged at 4500×g for 10min and pellets were dissolved in cheese whey at a concentration of 12.5X with respect to the initial volume of the cultures. Sodium alginate (40g/l) and the cryoprotectant (150g/l) was added to the culture in ratio 1:1 to reached final concentrations of 20g/l of alginate and 75g/l of cryoprotectant (Fig. 1C). Three types of capsules were made: capsules containing glycerol as cryoprotectant (Gly12.5X), capsules with cheese whey permeate (whose main component is lactose) (Per12.5X), and control capsules without cryoprotectant agents (Con12.5X). The capsules were kept at −20°C for 8h to solidify the contents. They were submerged in boiling water for three seconds, to remove the capsules from the mold, and then, they were submerged in a solution of 0.1M CaCl2 for 1h to allow alginate polymerization. Capsules were recovered by filtration, stored at −80°C during 8h, and freeze-dried (Fig. 1C). Half of the Gly12.5X, Per12.5X and Con12.5X were stored at 4°C and the rest were stored at RT. The experiments were performed in triplicate.
Storage of Gly12.5X capsules at −20°CGly12.5X capsules were made as described in Section “Production of capsules with cryoprotectants”. They were stored at −20°C for a period of 84 days. The experiments were performed in triplicate.
Determination of cell viabilityUncoated capsules were dissolved in a sodium citrate solution (10g/l) using a vortex9. Coated capsules were dissolved in a sodium citrate solution using a stomacher because of the hardness of the capsules. Dissolved capsules were serially diluted in phosphate-buffered saline (PBS), subsequently plated onto MRS agar, and then incubated in anaerobiosis for 72h at 37°C. The viability determinations were performed prior to the freezing step, immediately after freeze-drying, and sequentially at days 14, 28, 42, 56, 70 and 84 (Fig. 2). All determinations were done in triplicate.
Statistical analysisBacterial viability of RC, CC5X, CC12.5X, Con12.5X, Per12.5X and Gly12.5X, before and after freeze-drying, were measured with one-way ANOVA.
Bacterial viability of different type of capsules: RC, CC5X and CC12.5X, was evaluated by a factorial design of 3 (capsule types)×2 (with and without coating)×2 (RT and T4)×8 (day 0, 14, 28, 42, 56, 70, and 84).
Another factorial design was used to evaluate bacterial viability of Con12.5X, Per12.5X and Gly12.5X: 3 (cryoprotectants)×2 (RT and T4)×8 (day 0, 7, 14, 28, 42, 56, 70 and 84).
The bacterial viability through time of Gly12.5X stored at −20°C was evaluated by one-way ANOVA.
Differences between treatment means were tested for significance (p<0.05) by the Duncan's test.
For these analyses, software INFOSTAT version 2011 (InfoStat Group, FCA, National University of Córdoba, Argentina) was used.
ResultsEffect of capsule type and storage conditions on cell viabilityAll the factors analyzed (production method, storage temperature, and coating) showed different effects on bacterial viability through time.
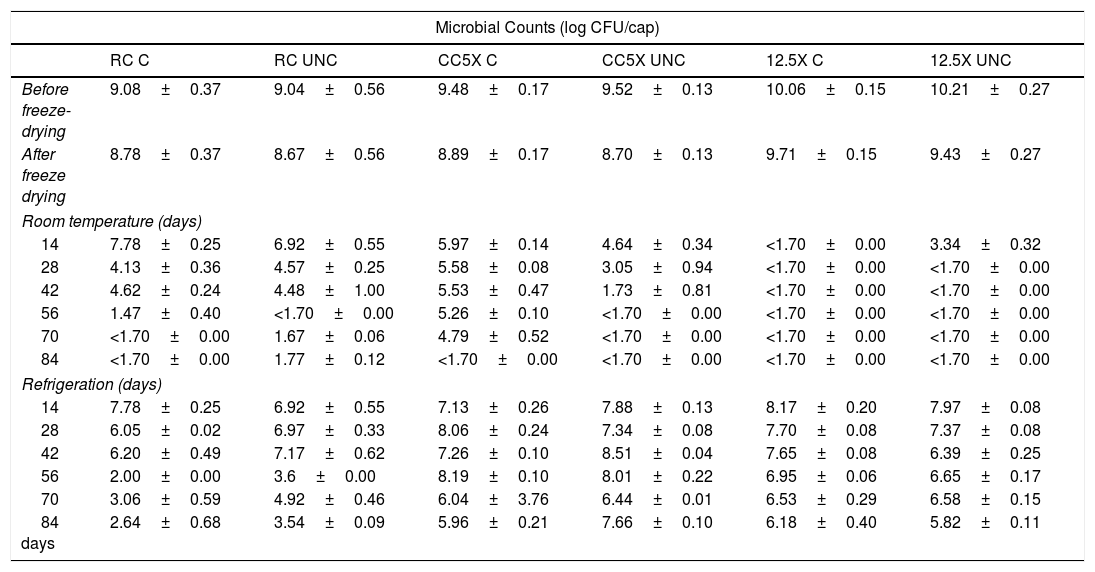
Initial bacterial counts were different for each type of capsule (p<0.001). The highest bacterial density was found in CC12.5X with 10.1log(CFU/capsule) and RC showed the lowest bacterial density with 9.06log(CFU/capsule). After the freeze-drying process, CC12.5X continued to show higher bacterial counts than RC and CC5X (p<0.001). No effect of the freeze-drying process was observed in RC and CC12.5X on bacterial viability (p>0.05). Conversely, a decrease in bacterial viability was observed in CC5X from 9.50log(CFU/capsule) to 8.79log(CFU/capsule) (p<0.001). The coating had no effect on viability in any of the capsule types evaluated (Table 1).
Microbial counts before and after freeze drying and during store at different temperatures (room temperature, refrigeration) for 84 days in different types of capsules.
| Microbial Counts (log CFU/cap) | ||||||
|---|---|---|---|---|---|---|
| RC C | RC UNC | CC5X C | CC5X UNC | 12.5X C | 12.5X UNC | |
| Before freeze-drying | 9.08±0.37 | 9.04±0.56 | 9.48±0.17 | 9.52±0.13 | 10.06±0.15 | 10.21±0.27 |
| After freeze drying | 8.78±0.37 | 8.67±0.56 | 8.89±0.17 | 8.70±0.13 | 9.71±0.15 | 9.43±0.27 |
| Room temperature (days) | ||||||
| 14 | 7.78±0.25 | 6.92±0.55 | 5.97±0.14 | 4.64±0.34 | <1.70±0.00 | 3.34±0.32 |
| 28 | 4.13±0.36 | 4.57±0.25 | 5.58±0.08 | 3.05±0.94 | <1.70±0.00 | <1.70±0.00 |
| 42 | 4.62±0.24 | 4.48±1.00 | 5.53±0.47 | 1.73±0.81 | <1.70±0.00 | <1.70±0.00 |
| 56 | 1.47±0.40 | <1.70±0.00 | 5.26±0.10 | <1.70±0.00 | <1.70±0.00 | <1.70±0.00 |
| 70 | <1.70±0.00 | 1.67±0.06 | 4.79±0.52 | <1.70±0.00 | <1.70±0.00 | <1.70±0.00 |
| 84 | <1.70±0.00 | 1.77±0.12 | <1.70±0.00 | <1.70±0.00 | <1.70±0.00 | <1.70±0.00 |
| Refrigeration (days) | ||||||
| 14 | 7.78±0.25 | 6.92±0.55 | 7.13±0.26 | 7.88±0.13 | 8.17±0.20 | 7.97±0.08 |
| 28 | 6.05±0.02 | 6.97±0.33 | 8.06±0.24 | 7.34±0.08 | 7.70±0.08 | 7.37±0.08 |
| 42 | 6.20±0.49 | 7.17±0.62 | 7.26±0.10 | 8.51±0.04 | 7.65±0.08 | 6.39±0.25 |
| 56 | 2.00±0.00 | 3.6±0.00 | 8.19±0.10 | 8.01±0.22 | 6.95±0.06 | 6.65±0.17 |
| 70 | 3.06±0.59 | 4.92±0.46 | 6.04±3.76 | 6.44±0.01 | 6.53±0.29 | 6.58±0.15 |
| 84 days | 2.64±0.68 | 3.54±0.09 | 5.96±0.21 | 7.66±0.10 | 6.18±0.40 | 5.82±0.11 |
RC: capsules made by reincubation method; CC5X: capsules made by concentration method 5X; CC12.5X: capsules made by concentration method 12.5X. C: coated capsules with chitosan; UNC: uncoated capsules; log CFU/cap: logarithm of colony forming unit per capsule.
The method used in the production of macrocapsules influenced bacterial viability (p<0.001) during storage. Moreover, storage temperature affected bacterial viability over time. Bacterial viability in all capsules stored at RT decreased below the SML before the 14th day of study (p<0.001). The chitosan coating had a protective effect on bacterial viability in CC5X stored at RT (p<0.001) (Table 1). Coated CC5X capsules had 4.79log(CFU/capsule) on day 70 while viability fell below the limit of detection on day 84. Uncoated CC5Xs maintained 3.05log(CFU/capsule) until day 28 and count was already below the detection limit on day 42. Although the capsules stored at 4°C decreased bacterial counts below 9log(CFU/capsule) in the first 14 days (p<0.001), they maintained a greater viability over time that the capsules stored at RT (p<0.001). During refrigeration, CC5X and CC12.5X maintained viability above SML until the end of the study, while RC only maintained viability above SML until day 42 (Table 1). At refrigeration storage, coating had no effect (p>0.05).
Effect of cryoprotectants in capsules stored at 4°C and RTIn order to improve bacterial viability during storage, neutralization of culture and addition of cryoprotectans was evaluated. For this study, CC12.5X were used, because this capsule type showed the highest initial bacterial count.
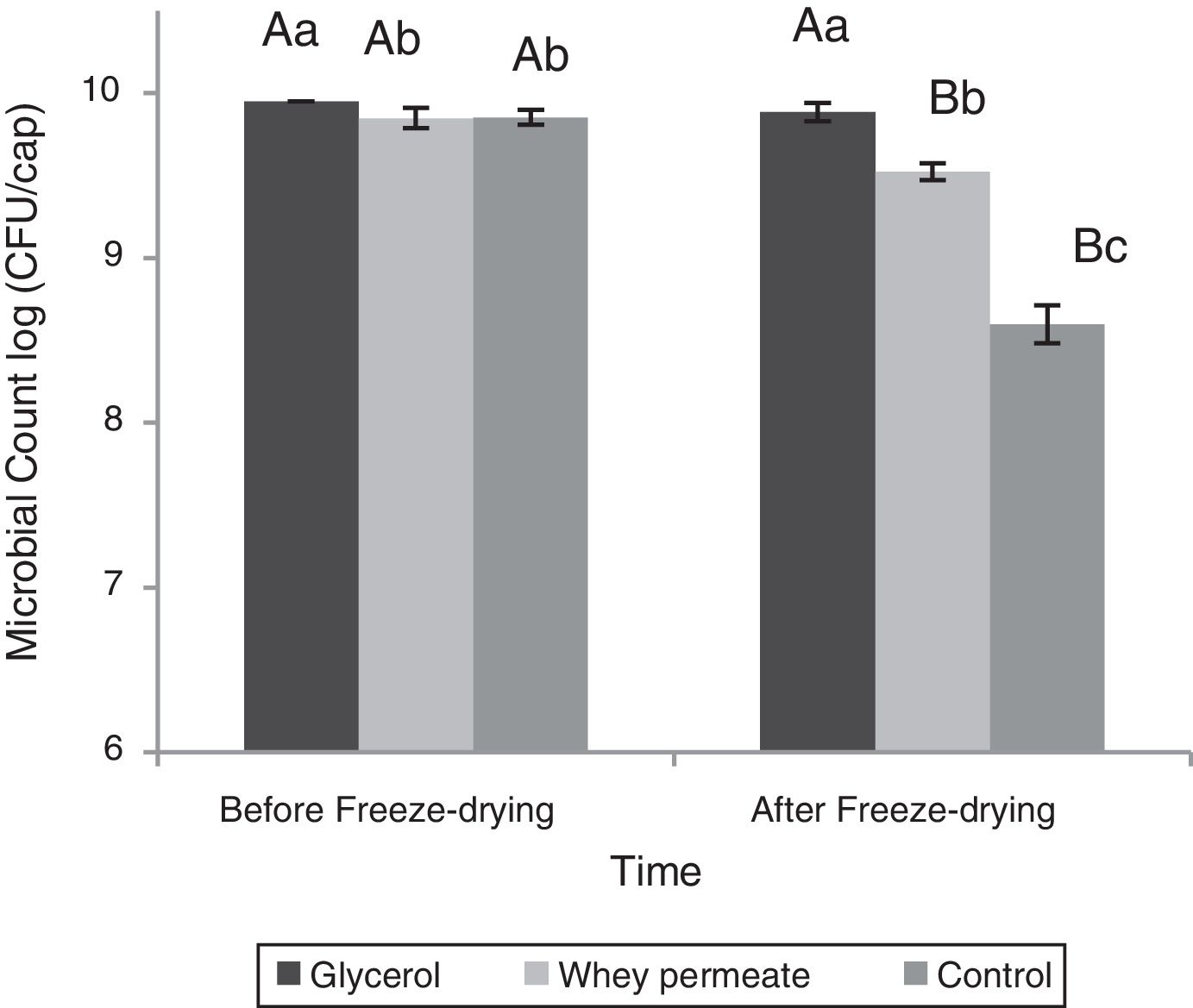
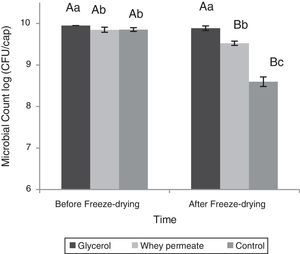
Capsules with glycerol maintained the initial viability after the freeze-drying process (p>0.05), whereas Per12.5X lost 0.3logCFU/cap and Con12.5X lost 1.25logCFU/capsule after the process (p<0.001) (Fig. 3).
Effect of cryoprotectants during the freeze-drying process. Lowercase letters represent the difference in microbial counts between capsules within each stage of the process. Capital letters represent difference counting in the capsules between the different stages of the process. Data represented as mean±standard deviation.
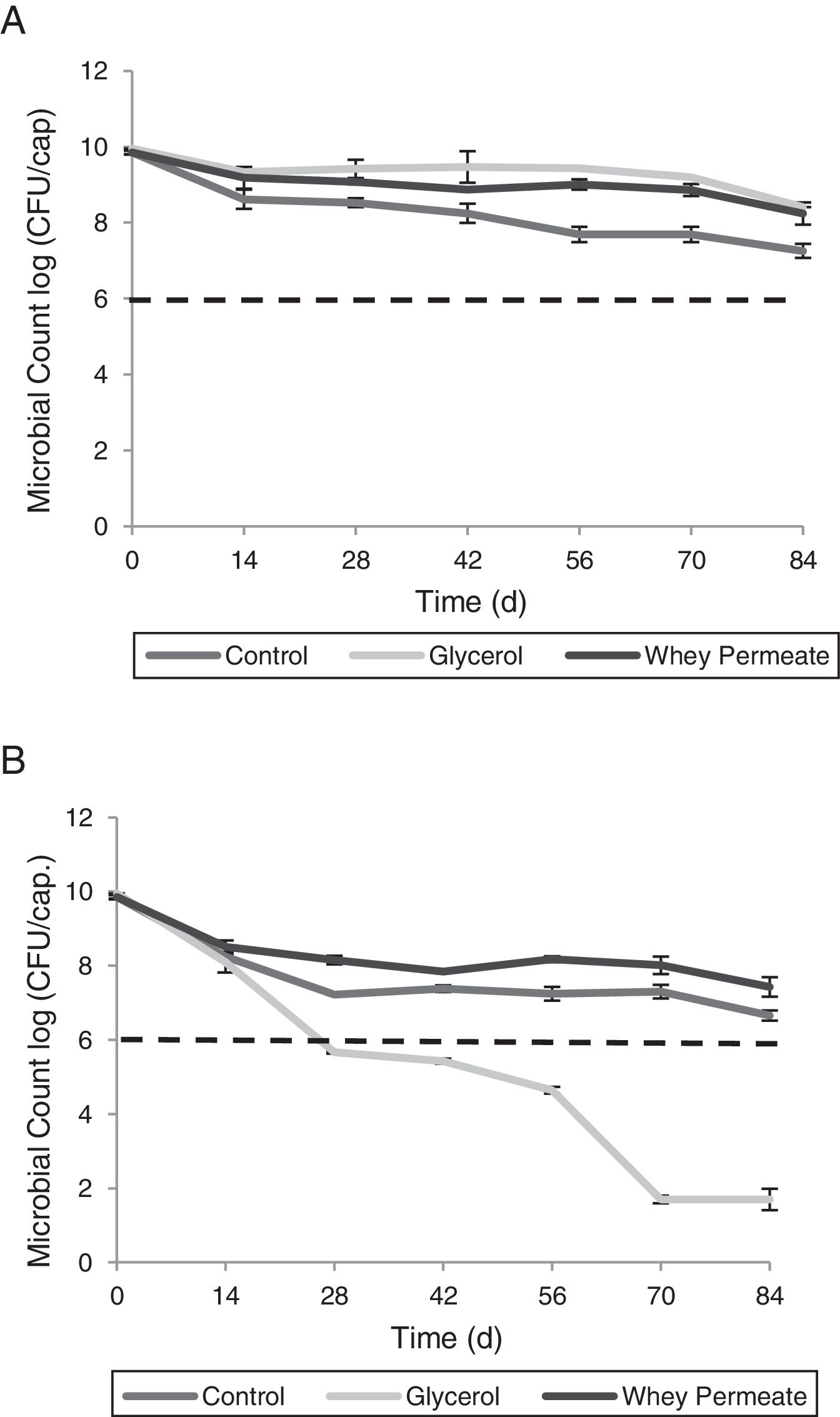
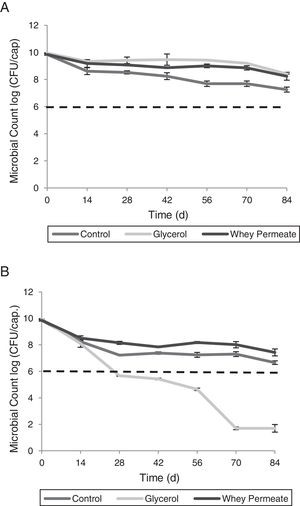
Storage temperature influenced bacterial viability depending on the selected cryoprotective agent (p<0.001). At 4°C, viability in all capsule types decreased in the first 14 days (p<0.001). Subsequently, Con12.5X showed a greater viability loss than Gly12.5X and Per12.5X over time (p<0.001). Con12.5X maintained values below 9log(CFU/capsule) during the studied period. In contrast, Gly12.5X exhibited counts above 9log(CFU/capsule) until day 70 and Per12.5X until day 56 of storage (p<0.001) (Fig. 4A).
All capsules stored at RT showed viability loss on day 14 (p<0.001). No differences were found between the capsules at this time (p>0.05) and all of them had a viability of less than 9log(CFU/capsule). Thereafter, Per12.5X and Con25X capsules maintained viability above the SML throughout the study, while Gly12.5X decreased bacterial viability (p<0.001) below SML (Fig. 4B). At the end of the study, capsules stored at RT reached the following values: Per12.5X=7.40log(CFU/capsule), Con12.5X=6.65log(CFU/capsule) and Gly12.5X=1.70log(CFU/capsule).
Protective effect of glycerol in capsules stored at −20°CGly12.5X capsules were selected for this test because they showed the highest bacterial counts after the freeze-drying process. Gly12.5X capsules maintained viability above 9logCFU during 84 days under these storage conditions (p>0.05).
The different types of capsules elaborated in this study are shown in Figure 5 and a summary of the ability to maintain the bacterial viability of each capsule type is described in Table 2.
Different types of coated (C) and uncoated (UNC) macrocapsules prepared in the present study. (A) Reincubated capsules (RC). (B) Concentrated capsules (CC). (C) Capsules with cryoprotectant (Gly12.5X: concentrated capsules 12.5X with glycine; Per12.5X: concentrated capsules 12.5X with whey permeate; Con12.5X: control capsules 12.5X without cryoprotectants).
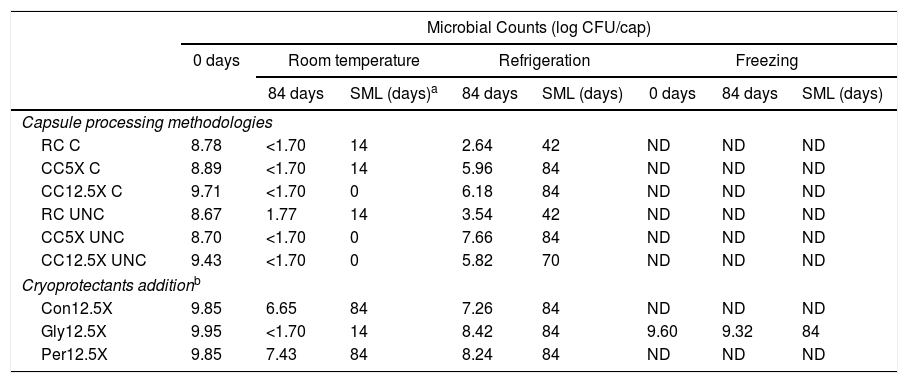
Microbial counts after freeze-drying (0 days) and after 84 days at different store conditions in the different types of capsules produced.
| Microbial Counts (log CFU/cap) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 days | Room temperature | Refrigeration | Freezing | |||||
| 84 days | SML (days)a | 84 days | SML (days) | 0 days | 84 days | SML (days) | ||
| Capsule processing methodologies | ||||||||
| RC C | 8.78 | <1.70 | 14 | 2.64 | 42 | ND | ND | ND |
| CC5X C | 8.89 | <1.70 | 14 | 5.96 | 84 | ND | ND | ND |
| CC12.5X C | 9.71 | <1.70 | 0 | 6.18 | 84 | ND | ND | ND |
| RC UNC | 8.67 | 1.77 | 14 | 3.54 | 42 | ND | ND | ND |
| CC5X UNC | 8.70 | <1.70 | 0 | 7.66 | 84 | ND | ND | ND |
| CC12.5X UNC | 9.43 | <1.70 | 0 | 5.82 | 70 | ND | ND | ND |
| Cryoprotectants additionb | ||||||||
| Con12.5X | 9.85 | 6.65 | 84 | 7.26 | 84 | ND | ND | ND |
| Gly12.5X | 9.95 | <1.70 | 14 | 8.42 | 84 | 9.60 | 9.32 | 84 |
| Per12.5X | 9.85 | 7.43 | 84 | 8.24 | 84 | ND | ND | ND |
RC: capsules made by reincubation method; CC5X: capsules made by concentration method 5X; CC12.5X: capsules made by concentration method 12.5X. C: coated capsules with chitosan; UNC: uncoated capsules; log CFU/cap: logarithm of colony forming unit per capsule. ND: not determined.
This study describes the production of freeze-dried probiotic macrocapsules destined for lactating calves with whey, sodium alginate, chitosan and cryoprotectants as components of the capsule matrix. Different types of capsule processing methodologies and combination of materials were evaluated to obtain macrocapsules with the highest initial cell density and to maintain the greatest viability over time under different storage conditions.
Production of high bacterial populations in alginate beads is possible by incubating the capsules in a nutrient medium8. However, the proliferation within the RC was not high enough to reach the bacterial load obtained by the concentration technique by centrifugation. Chew and Hadinoto11 suggest that capsules with higher cell density lost less viability during the freeze-drying process when no cryoprotectants were used. In this study, capsules made by the concentration technique with the lowest initial amount of bacteria (CC5X) decreased cellular density after the freeze-drying process. In contrast, the viability of capsules made with the same technique but with a higher bacterial density (CC12.5X) was not affected by the freeze-drying process. Likewise, capsules that had the highest initial counts (CC) were the ones that maintained the highest viability until the end of their storage period at 4°C, above SML. The capsules that had the lowest initial counts (RC) finished the storage period with counts smaller than SML. At RT storage, microorganisms show greater metabolic activity, production of acid metabolites and bacteriocins and even a loss of substrates20. Lower storage temperatures reduce harmful chemical reactions, such as fatty acid oxidation19, so that the best probiotic viability is achieved at refrigeration or freezing temperatures24,31 attaining a longer shelf life of the capsules20.
On the other hand, the effect of temperature was influenced by other factors such as capsule coating, culture neutralization prior to the preservation process and presence of cryoprotectants. Coated capsules have a kind of peripheral “skin” which increases thickness of the cover materials, which would improve the viability of the encapsulated cells, possibly due to better protection of bacteria against harmful environmental factors26. In this study, a coating effect was observed on capsule storage at room temperature. Under these conditions, the viability of coated capsules was greater than that of uncoated ones, but in both cases, the counts were lower than the SML from day 14. Some authors have reported that only chitosan-coated alginate capsules were able to maintain viability during storage1,38. However, in this study, under refrigeration conditions, coating did not improve viability during the storage period. Holkem et al.20 have reported that uncoated alginate capsules dried by freeze-drying have been able to maintain high microbiological counts during the storage period.
Neutralization of the culture medium at the end of the growing period had effects on bacterial viability. Bacterial viability can be improved by pH control, since the cells may lose their viability and activity when they are unable to maintain a near neutral intracellular pH in a low pH environment for a long period. On the contrary, the lack of pH control is beneficial for some strains, since the stress by pH generates an overproduction of proteins that make bacteria more resistant to subsequent processes21. In this study, two effects were observed. CC12.5X without neutralization did not decrease viability during freeze-drying, whereas the neutralized CC12.5X suffered a loss of viability of 1.25log(CFU/capsule), which indicates that neutralization did not have a positive effect during the process. However, the effect was positive during storage at room temperature, ending the study with a value higher than SML.
Cryoprotectants protect against damage caused by the freezing process, but they also generate a positive or negative effect on strain survival throughout storage. Sugars and their derivatives have been used as effective cryoprotectants for lactic acid bacteria since they have hydroxyl groups that provide protection against free radicals and by their water-binding capacity that prevents intracellular ice formation23. Numerous studies have shown that the addition of a protective agent, such as glycerol and lactose, improves the viability of probiotic bacteria stored at 4°C or −20°C13,15,24,27,29,36,37. This protective effect was observed in our study in the capsules preserved in refrigeration and freezing temperatures; however, in the case of the capsules containing glycerol stored at RT the effect was to the detriment of bacterial viability. Glycerol is very hygroscopic, therefore, if the freeze-dried product is not stored under conditions of low humidity, glycerol can absorb water from the environment, increase the water activity in microorganisms, and reduce their viability. Dianawati and Shah14 found that the use of glycerol had a tendency to increase water activity in the freeze-dried product and Kanmani et al.24 reported that glycerol has a lower protective effect during storage at RT. The higher water activity and storage temperature, the higher the rate of inactivation of microorganisms3,5,12. Conversely, for the capsules stored at RT the best results were observed for Per12.5X. The use of disaccharides alone or in combination with other cryoprotectants has shown improvements in viability in freeze-dried products stored at RT5. Gly12.5X kept up viability during the storage period when stored at −20°C. The cryoprotectant which is added to a highly concentrated cell suspension during freeze-drying undergoes a transition from the liquid state to the vitreous state. The glass transition temperature is the temperature at which the material undergoes a transition from the solid to the liquid state3. In general, freeze-dried products need to be stored below this transition temperature where they can maintain this state5. Water activity and glass transition temperature are critical factors that trigger adverse physicochemical reactions and cause bacterial inactivation. Glycerol and lactose are non-ionisable low molecular weight substances that cause amorphous and vitreous solidification rather than crystallization which would improve microbial viability during freeze-drying and storage at refrigeration and freezing temperatures22.
Several factors were evaluated in this study and the best conditions of production and storage of probiotic capsules could be determined. The production by concentration was the only that allowed to achieve cellular densities >9log(CFU/capsule). Another advantage of the concentration methodology is that it is faster than the reincubation methodology since it does not require incubation time. Another important determination is that the neutralization step of the growth medium prior to centrifugation generates greater bacterial viability over time. This is a step that generates high benefits and is low cost and no time-consuming. On the other hand, there is an interaction among other factors evaluated in this study: storage temperature, chitosan coating and type of cryoprotectant. It was determined that storage temperature has been a critical factor in the maintenance of viability. Only at refrigeration and freezing temperatures has it been possible to maintain viability above 9log(CFU/capsule) for some time. Under such conditions the presence of glycerol as a cryoprotectant has improved bacterial viability; however chitosan coating did not generate any benefits. Thus, capsules are uncoated, which simplifies the production process and decreases production costs.
A capsule with a bacterial density of 9log(CFU/capsule) would allow a dosage of 1 capsule per animal/day. Having the daily dose in a single capsule would reduce the complexity of administering the probiotic inoculum to the animals in the field. In turn, it reduces the costs of the materials that make up the matrix, because they would use less material/log bacteria. Another additional benefit is that capsules containing high densities of microorganisms would allow greater viability under simulated gastrointestinal conditions10 and this would help reach the colon in sufficient amounts to facilitate colonization.
ConclusionsThe probiotic inoculum containing L. casei DSPV318T and L. plantarum DSPV354T for lactating calves requires an administration of at least 9log(CFU)/day/calf to exert the probiotic effect. Capsules made by the concentration method by centrifugation, with neutralization of the medium and composed of sodium alginate, cheese whey and glycerol, reach the required cellular density. For storage over time, the refrigeration conditions maintain viability greater than 9log(CFU/capsule) for 70 days and under freezing conditions for at least 84 days. In future in vivo studies, these macrocapsules will be administered to calves to evaluate their efficiency as a probiotic vehicle by evaluating the protection exerted to probiotics undergastrointestinal conditions.
FundingThis study was part of the CAI+D Project financed by Universidad Nacional del Litoral, Santa Fe, Argentina.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors want to thank Laureano S. Frizzo, Lorena P. Soto, María V. Zbrun, and Marcelo L. Signorini, Research Career Members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and Jesica E. Blajman, Ayelén Berisvil, Analía Romero-Scharpen, Eugenia Rossler and Jorge A. Zimmermann, doctoral fellows from CONICET.