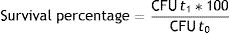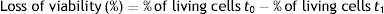The aim of this study was to evaluate the protective effect of the encapsulation of Limosilactobacillus reuteri DSPV002C in macrocapsules made from industrial materials during production, storage and under simulated gastrointestinal conditions in vitro and in vivo. The production of macrocapsules involved the evaluation of different wall materials (matrix), namely, gelatin and pregelatinized starch, different inoculums, matrix ratios, and diverse cryoprotectants (whey permeate and maltodextrin). The different macrocapsules were arranged in molds of similar size to pig pelleted food and lyophilized. Then, the viability of the macrocapsules was assessed over time during storage at different temperatures (freezing, refrigeration and room temperature) and atmospheres (vacuum and non-vaccum). The macrocapsules with 10% w/v gelatin+5% w/v pregelatinized starch, permeated (10%, w/v), with a 9:1 inoculum:matrix ratio (GS7.5P9), stored under freezing conditions and vacuum, exhibited the highest viability of L. reuteri DSPV002C (9.3 log CFU/cap until 210 d). Under simulated gastrointestinal conditions, the encapsulated inoculum showed less viability loss (0.58±0.09 log CFU/ml, 26.53%), compared to the free culture (1.56±0.16 log CFU/ml, 2.85%). Finally, by administering GS7.5P9 to pigs, the tolerance of the bacteria to the gastrointestinal environment was verified, with viable counts equal to or greater than 3.72 log CFU/g of fecal matter throughout the trial. In this study, a high-density carrier probiotic macrocapsule of L. reuteri DSPV002C was obtained, which displayed a long shelf life, a suitable shape to be included in pig feed and an adequate survival of viable cells at the site of action.
El objetivo de este trabajo fue evaluar el efecto protector de la encapsulación de Limosilactobacillus reuteri DSPV002C en macrocápsulas elaboradas con materiales industriales en el almacenamiento y en condiciones gastrointestinales, in vitro e in vivo. Para la producción de macrocápsulas, se evaluaron diferentes materiales de pared o matriz (gelatina y almidón pregelatinizado), diferentes proporciones de inóculo:matriz y diferentes crioprotectores (permeados de suero y maltodextrina). Las macrocápsulas fueron dispuestas en moldes de tamaño similar al del alimento peleteado para cerdos y se liofilizaron. Luego se estudió la viabilidad de las macrocápsulas en el tiempo almacenadas a distintas temperaturas (congelación, refrigeración y temperatura ambiente) y atmósferas (con vacío/sin vacío). Las macrocápsulas con gelatina al 10% (p/v)+almidón pregelatinizado al 5% (p/v) y permeado de suero (10% p/v), con una proporción inóculo:matriz 9:1 (GS7.5P9) y almacenadas a temperatura de congelación y al vacío, fueron las que mantuvieron la mayor viabilidad de L. reuteri DSPV002C (9,3 log UFC/cápsula hasta los 210 días). En condiciones gastrointestinales simuladas, el inóculo encapsulado mostró una pérdida de viabilidad menor (0,58±0,09 log UFC/ml; 26,53%) respecto del cultivo libre (1,56±0,16 log UFC/ml; 2,85%). Finalmente, mediante la administración de las cápsulas GS7.5P9 a cerdos, se comprobó la tolerancia de las bacterias encapsuladas al medio gastrointestinal, con recuentos de viables iguales o superiores a 3,72 log UFC/g de materia fecal durante todo el ensayo. En este estudio se obtuvo una macrocápsula probiótica portadora de alta densidad de L. reuteri DSPV002C, con una vida útil prolongada, una forma conveniente para ser incluida en el alimento de cerdos y una supervivencia adecuada de células viables en el sitio de acción.
The incidence of intestinal diseases is especially high in intensive rearing systems, where exposure to pathogens increases due to the confinement of large numbers of animals in small areas18,37. To avoid the detrimental effects of disorders in the microbiological balance of animals, antibiotic (AB) supplemented feed has been used to control pathogenic microorganisms. The continued use of AB in animal feed can lead to the development of resistant bacterial strains and the presence of residues in animal products22. The spread of AB-resistant microorganisms through the agri-food chain, as well as the presence of residues in food, has generated a demand from consumers and regulators to reduce or eliminate the use of AB.
An alternative to the use of AB in animal feed is probiotic supplementation, which can improve the intestinal balance and, therefore, the animal's natural defense against pathogens, resulting in higher profitability for farms25. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a benefit on the health of the host”4. Although the viability of the microorganisms is necessary to produce the probiotic effect, no consensus dose has been established due to the variation in beneficial effects exerted by each particular strain and doses administered in the different studies carried out. Furthermore, most of the studies do not report the dose administered to the animals, but rather the percentage incorporated into the diet35. Therefore, the dose cannot be estimated as the amount of feed consumed varies by animal and by category. In studies conducted with the strain Limosilactobacillus reuteri DSPV002C, beneficial effects have been found with doses between 109 and 1011 CFU/animal/day19,49.
Probiotics must survive in storage until administration as well as in gastrointestinal conditions39. These requirements raise the need to study the technological characteristics of the probiotic strains in terms of their viability over time and during their passage through the gastrointestinal tract by applying appropriate conservation methodologies. Therefore, various encapsulation techniques have been developed. Encapsulation can be defined as a physicochemical or mechanical process to trap a substance in a material and produce particles with micrometric diameters (microencapsulation), on a millimeter scale such as macrocapsules39. Macrocapsules have a lower surface-to-size ratio than microcapsules; thus, the amount of microorganisms on the surface directly exposed to adverse conditions in the gastrointestinal tract is smaller than in microcapsules2.
Materials used to encapsulate probiotic microorganisms must be food grade. Furthermore, they should be able to form a barrier that can protect the substance to be encapsulated9. Studies that consider alternative systems, such as biopolymers (gelatin and starch) for food protection, have increased significantly in recent years. These materials are completely biodegradable, often edible, and have few environmental effects17,31. Lyophilization is a technique used for the dehydration of heat-sensitive materials and bioactive compounds, since it uses low temperatures in the process16. Dairy product derivatives such as cheese whey proteins are widely used as cryoprotectants44,47. Maltodextrin has also been used for its cryoprotective effect36.
The presence of oxygen and the redox potential are among the most important factors that can affect the viability of probiotics during storage26. One of the techniques available to reduce oxygen content is vacuum packaging, a method used by several authors for the conservation of probiotics38,43. Another important factor is the storage temperature of the microorganisms, since the viability of the probiotic bacteria is inversely proportional to the storage temperature20.
The administration of probiotics to animals in farms requires a high concentration of microorganisms to get through gastrointestinal conditions and reach the site of action in order to exert effect3. Obtaining a probiotic capsule with a similar size and shape of the animal feed will allow to administer the inoculum to pigs directly mixed with food. The aim of this study was to evaluate the effect of encapsulation on the viability of L. reuteri DSPV002C during production, storage and in vitro/in vivo gastrointestinal conditions.
Materials and methodsInoculum productionA swine origin strain with in vitro and in vivo probiotic properties5,19 was used: Limosilactobacillus reuteri DSPV002C (GenBank accession number: GQ231436.1). L. reuteri DSPV002C was cultured in a fermenter (Sartorius Stedim Biotech, Goettingen, Germany) with 2% v/v of initial inoculum, and incubated 18h at 37°C. The culture medium consisted of 6% w/v cheese whey permeate (CWP) (Arla Food Ingredients, Porteña, Argentina), 0.8% w/v yeast extract (Oxoid, Basingstoke, Reino Unido), 0.0003% w/v MnSO4, 1% w/v casein peptone and 2% w/v dextrose. pH was adjusted to 6±0.2 with NaOH 6N. Afterwards, bacterial cells were harvested by centrifugation at 5000×g for 10min at 17°C, and cell pellets were washed with PBS twice.
Cell entrapmentTwo matrices composed of gelatin (PB LEINER PB GELATINS, Santa Fe, Argentina) and pregelatinized starch (“GLUTAGEL”, Glutal S.A. Esperanza, Argentina) were prepared. Gelatin was prepared at two concentrations, 10% w/v and 20% w/v, and heated at 70°C for 10min. Two solutions of pregelatinized starch were prepared at 5% and 20% w/v and heated at 70°C for 10min. Next, the gelatin-pregelatinized starch (GS) solutions were mixed in a 1:1 ratio until complete homogenization: 20% w/v gelatin+20% w/v starch (GS20), thus obtaining a 20% w/v final concentration matrix, and 10% w/v gelatin+starch 5% w/v (GS7.5), obtaining a 7.5% w/v matrix.
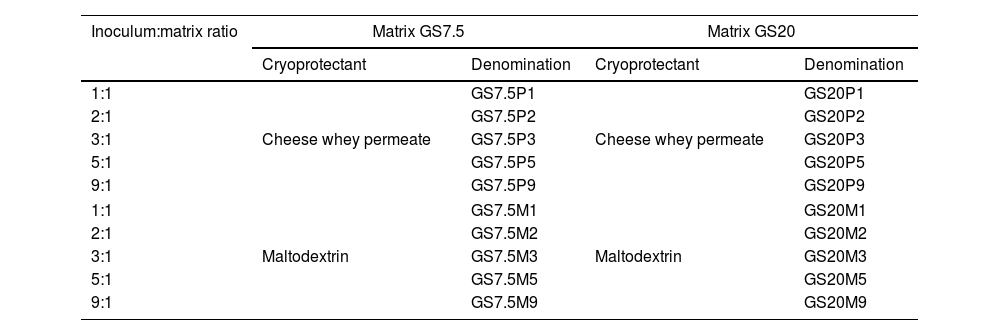
The matrices obtained were mixed with the inoculum (cell pellet) in five different proportions (Table 1). In addition, 10% w/v maltodextrin (corn maltodextrin “MALTRINA 15”. Glutal S.A. Esperanza, Argentina) or 10% w/v CWP final concentration were added as cryoprotectants (CP) to the different mixes. Table 1 summarizes the different combinations of inoculum:matrix+CP. The final mixtures were dispensed by extrusion into 10mm diameter silicone molds for the formation of the macrocapsules. The molds were then placed at −80°C for 18h and lyophilized at 0.044mbar (CHRIST® Alpha 1-4 LSCplus. Martin Christ, Osterode am Harz, Germany) for 18h. The macrocapsules were weighed prior to and after drying. The viability determination of the microorganisms in the macrocapsules was performed after drying by plate count. Disruption of the capsules and serial decimal dilutions were made in Ringer ¼ solution. Subsequently, the dilutions were plated on MRS agar and incubated at 37°C for 72h in anaerobiosis. All determinations were made in triplicate.
Different combinations of inoculum:matrix+cryoprotectants used for macrocapsules.
| Inoculum:matrix ratio | Matrix GS7.5 | Matrix GS20 | ||
|---|---|---|---|---|
| Cryoprotectant | Denomination | Cryoprotectant | Denomination | |
| 1:1 | Cheese whey permeate | GS7.5P1 | Cheese whey permeate | GS20P1 |
| 2:1 | GS7.5P2 | GS20P2 | ||
| 3:1 | GS7.5P3 | GS20P3 | ||
| 5:1 | GS7.5P5 | GS20P5 | ||
| 9:1 | GS7.5P9 | GS20P9 | ||
| 1:1 | Maltodextrin | GS7.5M1 | Maltodextrin | GS20M1 |
| 2:1 | GS7.5M2 | GS20M2 | ||
| 3:1 | GS7.5M3 | GS20M3 | ||
| 5:1 | GS7.5M5 | GS20M5 | ||
| 9:1 | GS7.5M9 | GS20M9 | ||
1:1 (50% inoculum+50% matrix); 2:1 (66.6% inoculum+33.3% matrix); 3:1 (75% inoculum+25% matrix); 5:1 (83.3% inoculum+16.6% matrix) and 9:1 (90% inoculum+10% matrix). GS7.5: gelatin 10% w/v+pregelatinized starch 5% w/v; GS20: gelatin 10% w/v+pregelatinized starch 10% w/v; P: whey permeate; M: maltodextrin.
For the in vivo test, a rifampicin-resistant L. reuteri DSPV002C was used to monitor the strain in fecal matter (FM). For this purpose, successive cultures of L. reuteri in MRS medium (Oxoid, Basingstoke, United Kingdom) were performed6, with increasing concentrations of rifampicin from 0.1μg/ml to 100μg/ml. The rifampicin-resistant strains were then propagated for 24h at 37°C in MRS broth. Next, macrocapsules GS7.5P9 containing the resistant strain were produced as previously described.
StorageThe macrocapsules selected from the cell entrapment assay were used. First, the macrocapsules were placed in amber glass containers and divided into two groups, vacuum packed and packed without vacuum. Vacuum in the glass containers was made at 0.065mbar (CHRIST® Alpha 1-4 LSCplus). Next, macrocapsules were stored under different temperature conditions: room (25±2°C), refrigeration (4°C) and freezing (−20°C) temperatures. The viability of the microorganisms in the macrocapsules was evaluated by plate count at 0, 35, 70, 105, 140, 175 and 210 days as previously described. All determinations were performed in triplicate.
Simulated gastrointestinal conditionsThe macrocapsules selected from the storage assay were used. Macrocapsules were kept for two weeks in freezing (−20°C) and vacuum-packed conditions as previously described. In order to obtain the free culture, a fresh culture of the L. reuteri DSPV002C strain was performed. The strain was grown in MRS broth in two consecutive passages at 37°C for 18h. After incubation, the strain was collected by centrifugation (3500×g for 5min), washed twice with Ringer's ¼ solution and recovered by centrifugation under the same conditions.
The study was performed in accordance with the methodology described by Wang et al., with modifications41. For simulated gastric juice (GJ), a suspension of pepsin was prepared in 0.2% NaCl w/v sterile at a final concentration of 0.35% w/v with pH adjustment to 3.0±0.2 with HCl. The solution was sterilized with a 0.22μm filter. For simulated intestinal juice (IJ), a sterile solution of 1.1% w/v NaCl with 0.2% w/v of NaHCO3 was used. Trypsin was added at 0.1% w/v and bile salts at 1.8% w/v. pH was adjusted to 8.0±0.2 with sterile NaOH. The solution was sterilized with a 0.45μm filter.
Three macrocapsules GS7.5P9 and 1ml of free culture were incubated with 9ml of GJ (pH=3±0.2) at 37°C with continuous agitation for 3h. Next, the sample was centrifuged (3500×g for 5min), and the pellet resuspended in 9ml of the IJ solution and again incubated for 3h at 37°C with continuous agitation. Viability of the free culture and the encapsulated strain was evaluated at 0, 1.5, 3, 4.5 and 6h by plate count and flow cytometry.
Determination of viability by plate countTo evaluate the viability of the microorganisms by plate count, the GJ or IJ was discarded by centrifugation. Then, the sediments of both types of samples were resuspended in Ringer's ¼ solution, and serial dilutions were made. Subsequently, dilutions were plated on MRS agar and incubated at 37°C for 72h in anaerobiosis. All determinations were made in triplicate.
The loss of viability was calculated by the following formula:
In addition, the survival percentage was calculated using the following formula:
where CFU is the colony forming units; t0 is the time 0 and t1 is the different incubation times (1.5h; 3h; 4.5h; 6h).Determination of viability by flow cytometryThe samples with microorganisms from the free culture and macrocapsules were centrifuged at 3500rpm for 5min, washed twice with 0.85% w/v NaCl and then adjusted to a concentration of 1×106CFU/ml. Subsequently, samples were stained using Syto9 and PI fluorochromes (Kit LIVE/DEAD BacLight Bacterial Viability, Molecular Probes, United States) according to the manufacturer's recommendations. A solution of the fluorochromes containing 1μl of Syto9 (3.34mM) and 0.5μl of IP (20mM) in 100μl of 0.85% w/v NaCl was prepared, and 20μl were placed in each bacterial inoculum. The samples were then incubated in the dark for 15min. Finally, the cells were acquired with an Attune NxT flow cytometer (Acoustic Focusing Cytometer A24860, Thermo Fisher Scientific). Data obtained were analyzed using specific software (FlowJo, TreeStar Inc., Ashland, USA).
The loss of viability was calculated by the following formula:
t0 is the time 0 and t1 is the different incubation times (1.5h; 3h; 4.5h; 6h).Administration of inoculum to animalsExperimental designTwenty piglets of both sexes (Sus scrofa domesticus), weaned at 28±1 d of life, were used. The weaned piglets were randomly divided into a probiotic group (PG) and a control group (CG) made up of 10 animals each, with two animals being placed per cage. The design of the experiment was in randomized blocks. The inoculum was administered from day 1 (28±1 d of life) until day 42 of the trial. The procedures used in this study were approved by the CICUAE (institutional committee for the care and use of experimental animals) of INTA CRBAN (Regional Center Buenos Aires North). Fecal samples were taken from six random piglets from the PG and six piglets from the CG, on days 0, 7, 14, 21, 28, 35 and 42 of the trial.
DietThe animals in both experimental groups were fed with commercial concentrate and received water ad libitum throughout the entire experiment. The diet was carried out with TEKNAL animal nutrition program, with Magnum Starter without antibiotics for piglets from 28±1 d to 35±1 d, then Magnum Initiator 300 in phase 3 until 45±1 d, and Magnum Breed 50 in phase 4 until 70 d. In addition, each piglet from the PG received the inoculum daily in the form of an oral capsule, every morning at the same time, thus ensuring a dose equal to or greater than 9.80 log CFU/animal/d. The administration of the inoculum was carried out from day 1 (28±1 d of life) until day 42 of the trial.
Sampling and microbiological analysisThe FM were collected in plastic bags by rectal massage or spontaneous defecation and kept refrigerated until their processing in the laboratory within 6h of being obtained. The samples were used to monitor the presence of the probiotic strain by microbiological counts. Microbial counts were made by serial decimal dilutions in Ringer's ¼ solution (Biokar, Beauvais, France) and plating in Petri dishes with specific media, LAMVAB medium (Lactobacillus Anaerobic MRS with vancomycin and bromocresol green)21, with the addition of rifampicin (100μg/ml) (LAMVABrif).
Statistical analysisFor cell entrapment, bacterial viability in macrocapsules was evaluated by a factorial design: 2 (matrices)×5 (proportions inoculum:matrix)×2 (CP) by using a factorial ANOVA and Duncan test. Bacterial viability under storage conditions within the macrocapsules was evaluated using a factorial design: 2 (matrices)×2 (CP)×2 (atmospheres)×3 (temperature), being analyzed using a factorial ANOVA of repeated measures and Duncan test. The difference in loss of bacterial viability in simulated gastrointestinal conditions was analyzed using an ANOVA for repeated measurements. One-way ANOVA was used for comparisons between groups at a specific time in the study. In addition, a one-way ANOVA with Duncan's test was performed to evaluate differences between times in the same group. The administration of the inoculum was analyzed using one-way ANOVA to establish differences within the same group throughout the trial. Results were expressed as the arithmetic mean±standard deviation (SD). A significance of p<0.05 was used for all the tests. For the analyses, software INFOSTAT version 2011 (Info-Stat Group, FCA, Universidad Nacional de Córdoba, Argentina) was used.
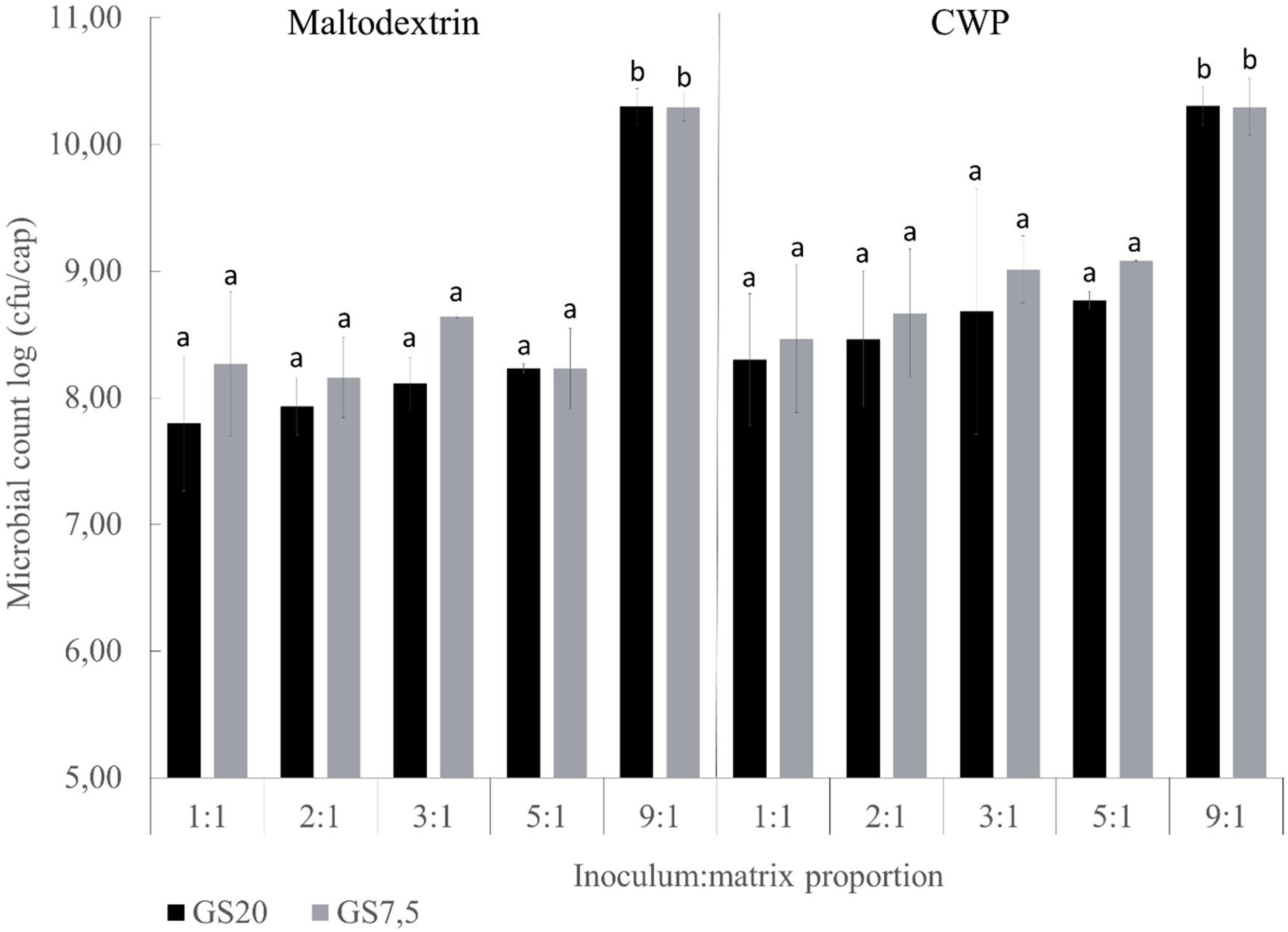
ResultsCell entrapmentViability of macroencapsulated L. reuteri DSPV002C is described in Figure 1. The weight of the macrocapsules was 0.35±0.05g and 0.09±0.01g prior to drying and after lyophilization, respectively. The type of matrix, GS20 and GS7.5 did not influence the viability of the inoculum (p=0.142). Considering the ratio of the inoculum:matrix, the macrocapsules with a 9:1 proportion had a higher viability of microorganisms (p<0.001). Regarding the two CP, CWP and maltodextrin, viability after the drying process was higher when using the CWP (p=0.008). For GS20, higher bacteria recovery was found in macrocapsules with 9:1 proportion (p<0.001). In macrocapsules GS20 with maltodextrin as CP, the counts were 2.49 log CFU/capsule higher in 9:1 inoculum:matrix proportion than in 1:1 (Fig. 1). Similarly, macrocapsules with CWP as CP showed a difference of 2.0 log CFU/cap between proportions 9:1 and 1:1. Macrocapsules with GS7.5 matrix, showed similar results to GS20, with a greater post-drying viability in GS7.5 in proportion 9:1 (p<0.001). When using maltodextrin, the viability was 2.02 log CFU/cap higher in proportion 9:1 than in 1:1. Macrocapsules GS7.5 with CWP as CP showed a difference of 1.83 log between the 9:1 and 1:1 proportions. The macrocapsules with a proportion of 9:1 inoculum:matrix were selected to continue with the following studies.
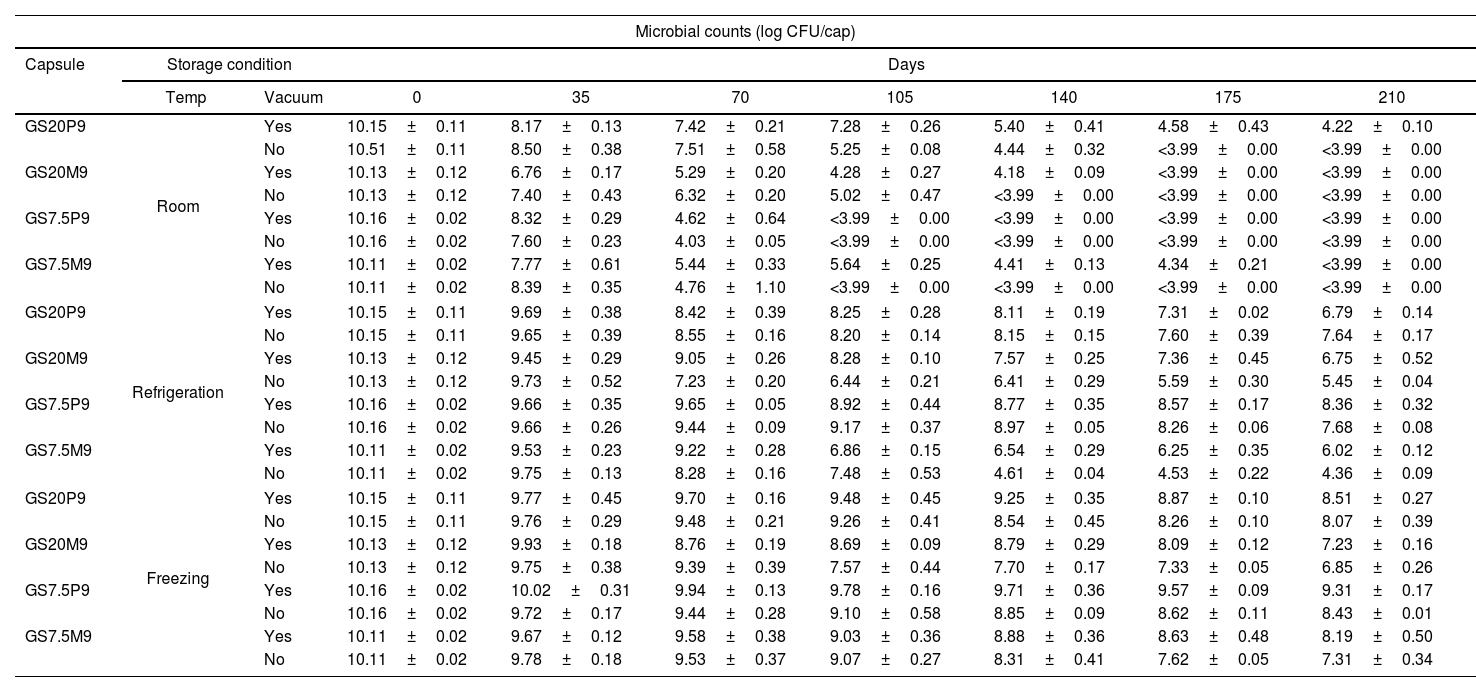
StorageThe results of storage viability are shown in Table 2. The use of CWP had a positive effect on the viability over time of the strain compared to maltodextrin (p<0.001). Macrocapsules stored at vacuum maintained higher viability than without vacuum (p<0.001). Storage temperature also influenced the cell viability. Macrocapsules kept in freezing temperature presented the higher viability, followed by refrigerated and room temperature, respectively (p<0.001). Furthermore, although macrocapsules GS20 and GS7.5 had a similar behavior (p=0.733), there was a matrix–storage temperature interaction (p<0.001). Capsules GS20 stored at room temperature presented greater viability than GS7.5, and in freezing temperature, GS7.5 showed better viability than GS20 (Table 2).
Microbial counts of L. reuteri DSPV002C in different types of capsules during storage in different conditions for 210 days.
| Microbial counts (log CFU/cap) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Capsule | Storage condition | Days | |||||||
| Temp | Vacuum | 0 | 35 | 70 | 105 | 140 | 175 | 210 | |
| GS20P9 | Room | Yes | 10.15±0.11 | 8.17±0.13 | 7.42±0.21 | 7.28±0.26 | 5.40±0.41 | 4.58±0.43 | 4.22±0.10 |
| No | 10.51±0.11 | 8.50±0.38 | 7.51±0.58 | 5.25±0.08 | 4.44±0.32 | <3.99±0.00 | <3.99±0.00 | ||
| GS20M9 | Yes | 10.13±0.12 | 6.76±0.17 | 5.29±0.20 | 4.28±0.27 | 4.18±0.09 | <3.99±0.00 | <3.99±0.00 | |
| No | 10.13±0.12 | 7.40±0.43 | 6.32±0.20 | 5.02±0.47 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | ||
| GS7.5P9 | Yes | 10.16±0.02 | 8.32±0.29 | 4.62±0.64 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | |
| No | 10.16±0.02 | 7.60±0.23 | 4.03±0.05 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | ||
| GS7.5M9 | Yes | 10.11±0.02 | 7.77±0.61 | 5.44±0.33 | 5.64±0.25 | 4.41±0.13 | 4.34±0.21 | <3.99±0.00 | |
| No | 10.11±0.02 | 8.39±0.35 | 4.76±1.10 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | <3.99±0.00 | ||
| GS20P9 | Refrigeration | Yes | 10.15±0.11 | 9.69±0.38 | 8.42±0.39 | 8.25±0.28 | 8.11±0.19 | 7.31±0.02 | 6.79±0.14 |
| No | 10.15±0.11 | 9.65±0.39 | 8.55±0.16 | 8.20±0.14 | 8.15±0.15 | 7.60±0.39 | 7.64±0.17 | ||
| GS20M9 | Yes | 10.13±0.12 | 9.45±0.29 | 9.05±0.26 | 8.28±0.10 | 7.57±0.25 | 7.36±0.45 | 6.75±0.52 | |
| No | 10.13±0.12 | 9.73±0.52 | 7.23±0.20 | 6.44±0.21 | 6.41±0.29 | 5.59±0.30 | 5.45±0.04 | ||
| GS7.5P9 | Yes | 10.16±0.02 | 9.66±0.35 | 9.65±0.05 | 8.92±0.44 | 8.77±0.35 | 8.57±0.17 | 8.36±0.32 | |
| No | 10.16±0.02 | 9.66±0.26 | 9.44±0.09 | 9.17±0.37 | 8.97±0.05 | 8.26±0.06 | 7.68±0.08 | ||
| GS7.5M9 | Yes | 10.11±0.02 | 9.53±0.23 | 9.22±0.28 | 6.86±0.15 | 6.54±0.29 | 6.25±0.35 | 6.02±0.12 | |
| No | 10.11±0.02 | 9.75±0.13 | 8.28±0.16 | 7.48±0.53 | 4.61±0.04 | 4.53±0.22 | 4.36±0.09 | ||
| GS20P9 | Freezing | Yes | 10.15±0.11 | 9.77±0.45 | 9.70±0.16 | 9.48±0.45 | 9.25±0.35 | 8.87±0.10 | 8.51±0.27 |
| No | 10.15±0.11 | 9.76±0.29 | 9.48±0.21 | 9.26±0.41 | 8.54±0.45 | 8.26±0.10 | 8.07±0.39 | ||
| GS20M9 | Yes | 10.13±0.12 | 9.93±0.18 | 8.76±0.19 | 8.69±0.09 | 8.79±0.29 | 8.09±0.12 | 7.23±0.16 | |
| No | 10.13±0.12 | 9.75±0.38 | 9.39±0.39 | 7.57±0.44 | 7.70±0.17 | 7.33±0.05 | 6.85±0.26 | ||
| GS7.5P9 | Yes | 10.16±0.02 | 10.02±0.31 | 9.94±0.13 | 9.78±0.16 | 9.71±0.36 | 9.57±0.09 | 9.31±0.17 | |
| No | 10.16±0.02 | 9.72±0.17 | 9.44±0.28 | 9.10±0.58 | 8.85±0.09 | 8.62±0.11 | 8.43±0.01 | ||
| GS7.5M9 | Yes | 10.11±0.02 | 9.67±0.12 | 9.58±0.38 | 9.03±0.36 | 8.88±0.36 | 8.63±0.48 | 8.19±0.50 | |
| No | 10.11±0.02 | 9.78±0.18 | 9.53±0.37 | 9.07±0.27 | 8.31±0.41 | 7.62±0.05 | 7.31±0.34 | ||
Macrocapsules: GS20P9: 10% w/v gelatin+10% w/v pregelled starch+10% w/v cheese whey permeate; GS7.5P9: 10% w/v gelatin+5% w/v pregelled starch+10% w/v cheese whey permeate; GS20M9: 10% w/v gelatin+10% w/v pregelled starch+10% w/v maltodextrin; GS7.5M9: 10% w/v gelatin+5% w/v pregelled starch+10% w/v maltodextrin.
After 35 d of storage at room temperature, GS20P9 with and without vacuum, GS7.5P9 with vacuum and GS7.5M9 without vacuum were the only macrocapsules that kept viability above 8 log CFU/cap (Table 2). The macrocapsules at room temperature lost viability, reaching counts lower than the minimum recommended dose (MRD) between 35 and 70 days of storage. This method was the least efficient for conservation. Macrocapsules stored in refrigeration showed a viability greater than 8 log CFU/cap at 70 d, except for GS20M9 without vacuum (Table 2). Until 140 d of storage, GS20P9 with and without vacuum kept viability above 8 log CFU/cap. Instead, GS7.5P9 with and without vacuum kept this viability at 175 d. Finally, GS7.5P9 with vacuum maintained viability above 8 log until 210 d, being the macrocapsule with the highest viability in refrigeration storage (Table 2). All the macrocapsules stored in freezing temperature maintained viability equal or above 9.3 log CFU/cap until 70 d. At 210 d, GS20P9 and GS7.5P9 with and without vacuum, as well as GS7.5M9 with vacuum, kept viability above 8 log CFU/cap (Table 2). The macrocapsule with the highest counts was GS7.5P9, with 9.31 log CFU/cap at 210 d. These were selected to continue the evaluation in the following phase.
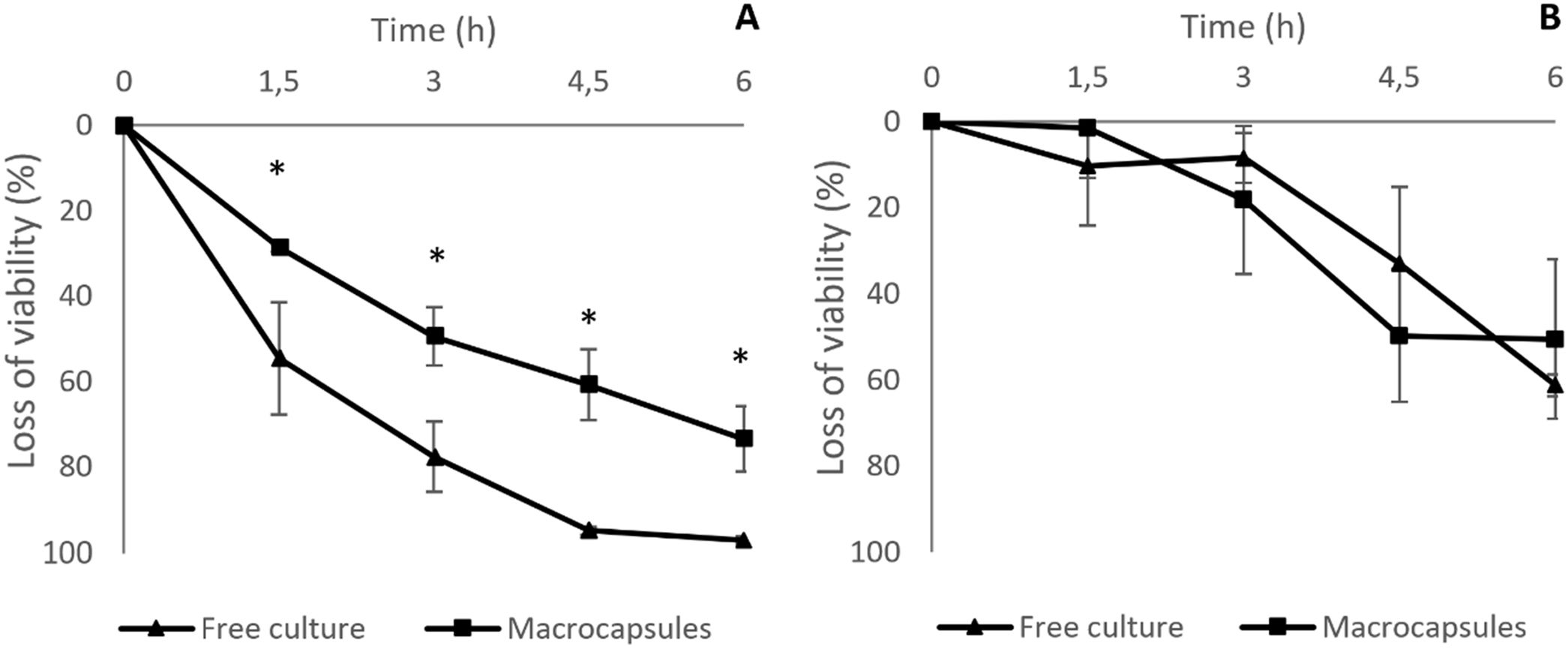
Simulated gastrointestinal conditionsThe loss of viability of L. reuteri DSPV002C determined by plate counting was less in macrocapsules than in free culture throughout the entire study (p<0.001). At t0, the counts were 10.68±0.05 log CFU/ml for the macrocapsules and 9.18±0.08 log CFU/ml for the free culture (Fig. 2A). After 1.5h of incubation in GJ, the macrocapsules had a loss of 0.14±0.01 log CFU/ml and the free culture 0.35±0.12 log CFU/ml, with a survival of 71.3% and 45.3%, respectively. At 3h of incubation, the viability loss was less for the macrocapsules (p=0.019), with 0.29±0.05 log CFU/ml and a survival of 50.53% compared to 0.66±0.04 log CFU/ml for the free culture, with a survival of 22.39% (Fig. 2A). At 4.5h and after a 1.5h incubation in IJ, the accumulated loss of viability was 0.41±0.06 log CFU/ml for the macrocapsules and 1.30±0.15 CFU/ml for free culture (p<0.001). This corresponds to a survival of 39.17% for macrocapsules and 5% for free culture. Finally, after 6h of incubation, the total loss of viability of the macrocapsules was 0.58±0.09 log CFU/ml vs. 1.56±0.16 log CFU/ml for the free culture. The survival percentage of the encapsulated microorganisms was 26.53% and 2.85% for the free culture (Fig. 2A).
Loss of viability of L. reuteri DSPV002C encapsulated in GS7.5P9 and without encapsulation (free culture), under simulated gastrointestinal conditions for 6h, evaluated by plate count and flow cytometry. GS7.5P9 macrocapsules: 10% gelatin+5% starch plus the addition of 10% cheese whey permeate as cryoprotectants. Loss of viability evaluated by plate count (A) and flow cytometry (B). Results are expressed as the mean±standard deviation. *Means differences between groups for the same time (p<0.05).
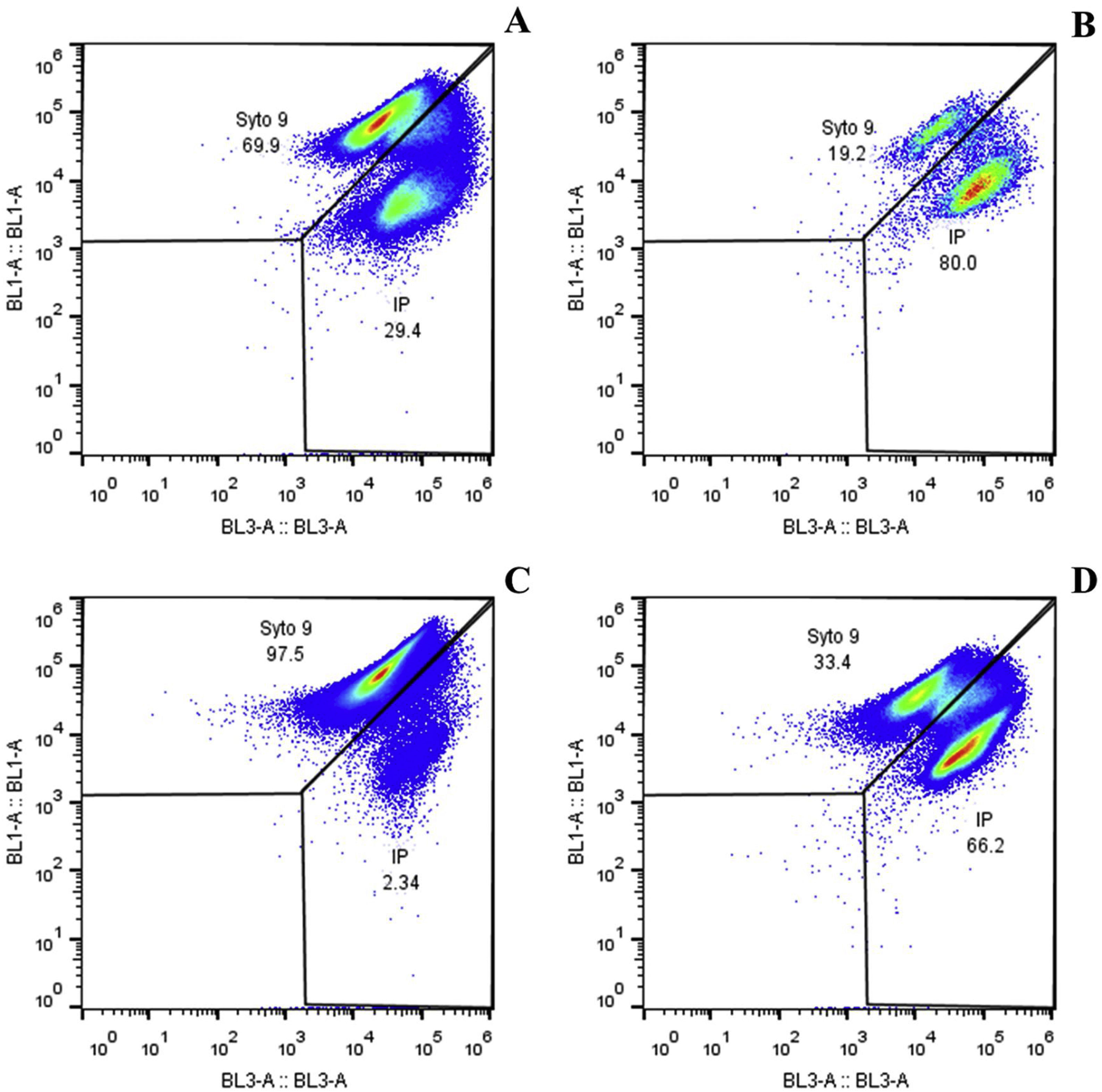
Two cell populations were identified in encapsulated microorganisms and in free culture through the viability analysis using flow cytometry. Live cells take fluorochrome Syto9 and were seen in the upper quadrant of dot plot BL1 vs. BL3 (Fig. 3), and dead or cell membrane damaged cells take fluorochrome IP and were seen in the right quadrant of the same graph. The percentage of live microorganisms in simulated gastrointestinal conditions was similar in free culture and in macrocapsules (p=0.808). At 3h, the macrocapsules lost 18±17.22% of viability while the free culture lost 8±5.74% compared to t0. After 6h of incubation, the macrocapsules showed a loss of viability of 50±18.52% and the free culture decreased its viability by 61±2.56% (Fig. 2B).
Viability of L. reuteri DSPV002C in macrocapsules and in free culture against simulated gastrointestinal conditions. Representative dot plot graphs showing the percentages of live and dead cells, in capsules and free culture, against simulated gastrointestinal conditions for 6h. BL1 (live bacteria stained with Syto9) vs. BL3 (dead bacteria stained with IP). (A) Macrocapsule 0h. (B) Macrocapsule 6h. (C) Free culture 0h. (D) Free culture 6h.
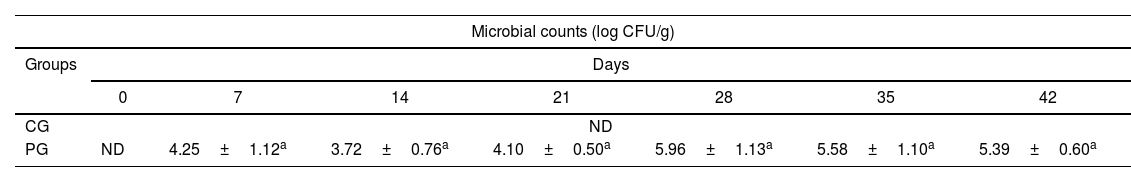
The recovery of L. reuteri DSPV002C in PG piglets was observed from the second to the last week of the test (Table 3). The probiotic strain recovery counts were between 3.72±0.7 and 5.96±1.1 log CFU/g of FM. In the control group, the administered strain was not recovered in any of the trial weeks. There were no differences in the probiotic inoculum counts between the evaluated days (p=0.243).
Counts of L. reuteri DSPV002C in fecal matter of piglets.
| Microbial counts (log CFU/g) | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Days | ||||||
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | |
| CG | ND | ||||||
| PG | ND | 4.25±1.12a | 3.72±0.76a | 4.10±0.50a | 5.96±1.13a | 5.58±1.10a | 5.39±0.60a |
CG: control group; PG: probiotic group; ND: not detected. Data are expressed in log CFU/g of fecal matter and correspond to the mean±standard deviation. Lowercase letters represent the difference in microbial counts between days.
The encapsulation of probiotic microorganisms fulfills more of a protective function than a controlled release function11, and the viability of the encapsulated microorganisms will depend in part on the physicochemical characteristics of the capsule9. In order to incorporate the encapsulated microorganisms in the pigs’ diet together with the feed, it is necessary for the macrocapsules to have a size similar to that of the feed pellets. The use of molds allowed to generate a similar size to that of the pelletized feed for pigs. In this way, a product suitable for incorporation and mixing with the animals’ diet was obtained without interfering with their normal feeding, thus becoming an advantage for their implementation on farms.
Evaluation of different inoculum and matrix proportions allowed us to select a macrocapsule with high concentrations of microorganisms. It is necessary to start from a high concentration of microorganisms per macrocapsule to ensure an adequate viable bacterial load until its administration to the animals with the MRD of 108 CFU/d. Cryoprotective agents can be added during the growth of microorganisms or before freezing or drying said microorganisms33. In addition to being added before drying, CWP was already part of the culture medium as opposed to maltodextrin, which was added before drying and was not part of the culture medium for biomass development. This could have some influence on the better performance of CWP as a cryoprotectant against maltodextrin.
Packaging in a modified atmosphere offers the possibility of extending the shelf life of products through the reduction of the oxygen levels to avoid toxicity and death of microorganisms13. Capsules stored without oxygen protection may have been affected because oxygen affects microorganisms in different ways; this is due to the production of toxic peroxides in the presence of oxygen and the production of free radicals by oxidation of other components23. In this work, a decrease in viability was observed in all the macrocapsules throughout the 210 days of the storage trial. Regarding storage temperatures, a greater loss of viability was found under refrigeration conditions and room temperature than in freezing conditions. Pedroso et al. attributes the loss of viability at refrigeration and room temperature to the fact that microorganisms are metabolically active, with the production of metabolic acids, bacteriocins and the absence of substrates34. In our study, the most efficient method for the conservation of macrocapsules was freezing, followed by refrigeration; the least efficient method was conservation at room temperature. Low temperature storage of probiotics has been effective for a long time; for the maintenance of lyophilized probiotics, temperatures of −18°C are recommended7.
In order to reach the intestine in adequate amounts and exert its effect on the host, it is necessary for probiotic microorganisms to survive the acidic environment of the stomach28. To resist gastrointestinal conditions, polysaccharides and proteins are used as protective agents that provide a physical barrier to bacteria against acid and bile30. In plate count determinations, the viability in GJ and IJ of L. reuteri was greater in macrocapsules than in free culture, thus observing the protective effect of encapsulation in the environment of the stomach and intestine. Other studies that have used gelatin and starch to encapsulate probiotic bacteria also reported positive protective effects during gastrointestinal digestion1,29.
In flow cytometry, we found results that were not consistent with those found in the traditional technique because the protective effect of the macrocapsule could not be evidenced. The fact that the standard technique and flow cytometry did not show similar results at all the studied times could be explained by those bacteria that are in a viable non-cultivable state. Viability determination by flow cytometry is based on membrane integrity and, therefore, this technique may give a different response to plate count. The term viability refers to the ability to multiply and be cultivable, which is an essential condition for determining viable cells by plate counting. However, in flow cytometry, the definition of viability is related to the ability of a cell to perform various aspects of metabolic, physiological and genetic functionality, maintaining the degree of structural and morphological integrity24 but also including those cells that have lost the ability to multiply, a state that is described as viable but non-cultivable (VBNC)48. This is a strategy used by bacteria to enter a state of very low metabolic activity, which allows them to survive stress conditions. These bacteria can regain the ability to grow and multiply if given the right conditions and environment14. Only a limited number of studies have compared bacterial viability using plate count and flow cytometry. Wilkinson indicates that, in the production of probiotics and subsequent processes, there are many factors that influence viability and survival characteristics that can affect the relationship between cytometric analysis and plate counts45. Among the main factors, the handling of process variables during fermentation, such as pH, the degree of oxygen incorporation, as well as exposure to subsequent processing such as heat treatments, are cited. Some authors have found a high correlation between the plate counts and the results obtained by flow cytometry since they used fresh cultures8,27, while others have found a low correlation since they studied microorganisms that had gone through some type of processing15,46. In our work, we found differences between the reproductive capacity of encapsulated and free cells. The environment had a stressor effect on the free cells, thus causing a large number of them to go into a VBNC state. Considering the differences between the methods used to determine viability, it should be studied whether the cells in the VBNC state can effectively exert their probiotic effect if the environmental conditions are favorable. Encapsulation protects the cells from entering the VBNC state, which would ensure that when they reach the site of action, they can exert their probiotic effect.
Regarding the in vivo test, in order to evaluate the recovery of the probiotic strain, the monitoring method of resistant clones using LAMVABrif was adequate for our purpose. After administration, L. reuteri DSPV002C was recovered from the FM indicating that, as in the in vitro test, the strain went through gastrointestinal conditions and then reached the site of action. Matijasic et al. found variability between animals when analyzing feces recovered after administering two probiotic strains32. Some authors indicate that the fecal counts of probiotics decrease even with constant administration10,40. In our study, the recovery of the probiotic in the FM was constant without differences between days. Encapsulation techniques have been shown to be effective in protecting different probiotic microorganisms under simulated gastrointestinal conditions12. However, there are few studies that evaluate the protective capacity of encapsulation in in vivo digestive conditions. Wang et al. administered an encapsulated probiotic strain of L. reuteri to pigs, and compared it with the administration of a non-encapsulated strain, thus obtaining an increase in shedding in FM in the animals with the encapsulated strain42. The production of macrocapsules with a high density of microorganisms allowed us to ensure the passage through gastrointestinal conditions to the site of action, which was reflected in the recovery of the strain in the FM.
ConclusionMacrocapsules with an inoculum:matrix ratio of 9:1 allowed us to obtain a product with a quantity of microorganisms higher than the MRD. Regarding cryoprotectants, CWP was better than maltodextrin in protecting the probiotic inoculum in the lyophilizate process. Of all the storage conditions, the macrocapsules kept frozen, with PS and vacuum packed, were the ones that showed the best viability up to 210 d of storage. The GS7.5P9 macrocapsules were able to protect the L. reuteri DSPV002C inoculum, showing less loss of viability than the free culture after 6h of incubation under simulated gastrointestinal conditions. Viability by flow cytometry did not agree with the results obtained by plate counting under simulated gastrointestinal conditions. Through this study, a macrocapsule with high bacterial densities was obtained with a long useful life and with the appropriate shape to be added to pig feed. After administration to the animals, the strain was recovered, thus demonstrating it had reached the site of action in the gastrointestinal tract. In the future, it would be interesting to carry out other tests where the probiotic effect on different parameters (e.g. growth performance, immunology, health status) in the animals are evaluated.
FundingThis study was part of a CAI+D Project financed by Universidad Nacional del Litoral, Santa Fe, Argentina.
Conflict of interestThe authors declare that they have no conflicts of interest.