The objective of the present study was to explore the influence of dietary supplementation with a mixed additive (MA) containing a probiotic and anti-mycotoxin (Saccharomyces cerevisiae RC016 and Lactobacillus rhamnosus RC007) and its interaction on the performance and health (biochemistry and liver/intestine histopathology) of broilers fed diets contaminated with aflatoxin B1 (AFB1) at 506000±22.1ng/kg. The MA contained S. cerevisiae RC016 (1×107cells/g) and L. rhamnosus RC007 (1×108cells/g) in relation 1:1. A total of sixty-one-day-old Cobb broilers were randomly allocated into four treatment groups with three replicates of 5 birds each for a five-week-old feeding experiment. The experimental diet for each treatment (T) was formulated as follows: T1, a commercial diet (CD); T2, CD+AFB1; T3, CD+0.1% MA; T4, CD+AFB1+0.1% MA. The MA improved (p<0.01) production parameters (weight gain, conversion rate, and carcass yield) and reduced (p<0.01) the toxic effect of AFB1 on the relative weight of the livers. In addition, the macro and microscopic alterations of livers and the possible intestinal injury related to histological damage in the presence of mycotoxin were reduced. The use of probiotic MA based on S. cerevisiae RC016 and L. rhamnosus RC007 in animal feed provides greater protection against mycotoxin contamination and is safe for use as a supplement in animal feed, providing beneficial effects that improve animal health and productivity. This is of great importance at the economic level for the avian production system.
El objetivo del presente trabajo fue estudiar la influencia de la suplementación dietética de un aditivo mixto (AM) de probióticos y antimicotoxinas, y su efecto sobre el rendimiento y la salud (bioquímica e histopatología de hígado/intestino) de pollos de engorde alimentados con una dieta contaminada con aflatoxina B1 (AFB1), a una dosis de 506.000±22,1ng/kg. El AM contenía Saccharomyces cerevisiae RC016 (1×107 células/g) y Lactobacillus rhamnosus RC007 (1×108 células/g) en una relación 1:1. Un total de 60 pollos de engorde (Cobb) de un día de edad se asignaron al azar en cuatro grupos de tratamiento con tres réplicas de 5 aves cada una, durante 5 semanas, a saber: T1, dieta comercial (DC); T2, DC+AFB1; T3, DC+0,1% AM; T4, DC+AFB1+0,1% AM. El AM mejoró (p<0,01) los parámetros de producción (ganancia de peso, tasa de conversión y rendimiento de la canal) y redujo (p<0,01) el efecto tóxico de la AFB1 sobre el peso relativo de los hígados. Además, se disminuyeron las alteraciones macro y microscópicas del hígado y la lesión intestinal posiblemente relacionada con el daño histológico en presencia de la micotoxina. El uso de probióticos y antimicotoxinas a base de S. cerevisiae RC016 y L. rhamnosus RC007 en la alimentación animal proporciona mejora la protección contra la contaminación por micotoxinas y es seguro para su uso como suplemento en la alimentación animal, con efectos beneficiosos que mejoran la salud y la productividad animal. Esto es de gran importancia en términos económicos para el sistema de producción aviar.
The poultry industry is one of the fast-growing sectors among the different agricultural sectors. The need to increase the efficiency of productive systems, not only the production of meat but also competitiveness, has given rise to an intensification process of these systems, in which different challenges such as stress, antibiotics and modern breeding practices have been presented. For several decades, animal growth-promoting antibiotics (GPA) were used in sub-therapeutic doses as additives to improve animal health and well-being, as well as to increase growth, to improve meat production through increased food conversion, and disease prevention3,13,32,41. The abusive use of GPA and the associated selection pressure decreased therapeutic efficacy and led to the emergence of populations of antibiotic-resistant microorganisms. Due to the prohibition of the use of GPA, there is a growing demand for alternative additives that provide benefits for animal health and growth worldwide.
A wide variety of non-therapeutic alternatives that can replace antibiotics such as probiotics, prebiotics, enzymes, organic acids, immunostimulants, bacteriocins, bacteriophages, phytogenic additives, nanoparticles, and essential oils were considered35,36. Probiotics are gaining acceptance as alternatives to GPA to improve production efficiency. They are mono or mixed cultures of living organisms that when administered in adequate amounts confer a health benefit to the host20. Probiotics can be administered alone or in combination with other additives in food or water. In addition, there are many studies that have demonstrated their ability to interact with food contaminants and thus, reduce the amount that reaches the bloodstream to reduce their bioavailability5,14,42.
A variety of bacteria (Bacillus, Bifidobacterium, Enterococcus, Lactobacillus, Streptococcus and Lactococcus spp.) and yeasts (Saccharomyces spp.) were tested as probiotic additives in poultry. Their use should maintain good health and not affect the environment. Moreover, they should improve performance characteristics that include, among others, improved feed conversion ratio, average daily weight gain, egg production, carcass composition, reproductive performance of breeding females, and also decrease the incidence of diseases, exhibiting a growth-promoting effect1,11,12. The application of yeasts in feed, and their status as microorganisms generally recognized as safe (GRAS) make them an adequate basis for strategies designed to reduce oral exposure to chemical contaminants such as mycotoxins. The animal feed industry needs to produce high nutritional value and microbiological quality feeds to ensure good animal health and performance, while replacing the use of GPA.
Previous studies allowed the selection of strains of Saccharomyces cerevisiae and Lactobacillus rhamnosus native to the intestine of animals and silage, with different mechanisms of action to reduce the bioavailability of mycotoxins. In in vitro tests, yeast demonstrated the ability to adsorb different mycotoxins at the cellular level6,8,9,17. The dynamics of AFB1 adsorption and desorption by L. rhamnosus in a simulated gastrointestinal tract model was also described, postulating a possible degradation thereof22. The probiotic potentialities were also evaluated individually. The yeast showed in vitro assays, inhibition and coaggregation of pathogens, adhesion to intestinal epithelial cells7,37 and in vivo assays26,39,40 and resistance to passage through the simulated gastrointestinal tract22. Lactic acid bacteria (LB) demonstrated the absence of antibiotic resistance genes in vitro18. In challenges, it demonstrated a strong stimulation of the immune system in rats19,24 and also in an ex vivo porcine model25. These microorganisms were obtained at the bioreactor scale using statistical experimental designs to optimize their performance21. The mixed formulation of S. cerevisiae RC016 and L. rhamnosus RC007 at concentrations of 105 and 106cells/ml and its cell-free extracts did not show toxicity in Vero cells, and its administration in healthy BALB/c mice did not produce overstimulation of the immune system while maintaining the normality of the determined immunological parameters22. Investigating the effective use of feed additives containing yeast strains and/or LB in a mixture as mycotoxin adsorbents and/or degraders with probiotic properties is a promising alternative.
Based on the above, the objective of the present work was to study the influence of dietary supplementation with a mixed additive (MA) containing a probiotic and anti-mycotoxin (S. cerevisiae RC016 and L. rhamnosus RC007) and their interaction on the performance and health (biochemistry and liver/intestine histopathology) of broilers fed diets contaminated with aflatoxin B1 (AFB1) contaminated diets.
Materials and methodsThe working protocol and the techniques used comply with the regulations of the Subcommittee on Animal Bioethics under the Ethics Committee of Scientific Research, as established in Resolution 253/10 of the Superior Council of the National University of Rio Cuarto.
MicroorganismsS. cerevisiae RC016 was isolated from the animal ecosystem and identified by molecular techniques through DNA extraction and 18S rRNA and 28S rRNA amplification and analysis, comparing sequences with the Basic Local Alignment Search Tool (BLAST) within the National Center for Biotechnology Information (NCBI) database7.
L. rhamnosus RC007 was isolated from maize silage and identified from both the fermentation pattern (API 50 CHL test) and the 16S rRNA gene sequence18. These strains are deposited in the culture collection of the Industrial Microbiology Laboratory of the National University of Rio Cuarto collection center, located in Río Cuarto, Córdoba, Argentina.
Yeast and bacterial biomass production and formulationS. cerevisiae RC016 biomass was obtained from a 24-h culture in yeast–peptone–dextrose (YPD) broth, supplemented with 1g of PO4H2K/l in a BioFlo 2000 fermentor (New Brunswick Scientific Co., Inc, Enfield, CT, USA) operated at 4×g at 28°C, for 12h and 1.5vvm aeration. The pH value was adjusted to 5 with 6M NaOH. The working volume was 4l.
L. rhamnosus RC007 culture conditions were 3l of optimized culture medium developed with a low-cost substrate (commercial refinery syrup), stirring 4×g at 37°C for 24h and 10% inoculum (v/v). The concentration of dissolved oxygen at the beginning of the experiment was 0%. Foam production was controlled by the addition of antifoam 289 (Sigma-Aldrich, St. Louis, MO, USA). pH was maintained between 6.5 and 7, with the addition of 18N H2SO4 or Na2CO3 20% w/v.
The biomass obtained at the end of the fermentation was centrifuged at 1000×g at 4°C for 10min. The concentrated pellet was resuspended in the same volume of cryoprotectant (10% skim milk plus 5% yeast extract, for the yeast and 10% skim milk only for the bacteria) and stored at −80°C. The lyophilized formula (1g) was hydrated and viability was confirmed. Finally, the mixed additive (MA) formulation was made mixing the lyophilized microorganisms (1:1) as follows: S. cerevisiae RC016 at 1×107cells/g and, L. rhamnosus RC007 at 1×108cells/g and then, 0.1% MA (0.1g MA per 100g commercial diet – CD – described below) was used for the in vivo trial.
Aflatoxin B1 productionSufficient AFB1 was produced to contaminate the experimental feed, according to the methodology proposed by Gonzalez Pereyra et al.26 from the culture of the reference strain Aspergillus parasiticus NRRL2999. The AFB1 content of the resulting powder was quantified by high performance liquid chromatography (HPLC) following the methodology described by Trucksess et al.43. Analyses were performed in triplicate. The AFB1 contaminated powder was then added to the premix to reach a final concentration of 506000±22.1ng/kg. The diet without AFB1 addition had 22.15±1.15ng/kg natural AFB1 contamination.
Experimental designAnimalsThis experimental study was repeated twice. One-day old male Cobb 500 broilers (0.060±0.020kg, total n=60) vaccinated against Marek's disease obtained from a commercial hatchery were used. The broiler chickens were stabilized for a period of one week. Water and feed were provided ad libitum. They were then randomly housed in stainless steel metabolic cages (3 replicates of 5 animals each/cage). The broiler chickens were raised in cages instead of floor pens to reduce the operational cost. Each cage contained a tube feeder and drinker and was covered with fresh chips. Animals were kept under 20h fluorescent light and 4h of obscurity. The temperature was adjusted daily to maximize the comfort of the birds.
DietsThe broiler chickens were fed a commercial starter diet from hatch until 28 d of age (4 weeks) and a commercial grower diet from 29 to 35 d of age (4–5 weeks). The diet was corn and soy-based, meeting the rules and regulations of the National Research Council (NRC) requirements for broilers15. The chickens were fed with the experimental diets from day 1 until they were slaughtered at 35 days of age (Table 1 supplementary material). The experimental diets for each treatment (T) were formulated as follows: T1: commercial diet (CD); T2: CD+AFB1 (506000±22.1ng/kg); T3: CD+AFB1 (506000±22.1ng/kg)+0.1% MA (1:1) from lyophilized microorganisms: S. cerevisiae RC016 (1×107cells/g) and L. rhamnosus RC007 (1×108cells/g) and T4: CD+0.1% MA.
Productive parameter determinationBroiler chickens were weighed weekly during the experimental period (five weeks) and their feed intake was calculated. Moreover, animals were monitored daily for morbidity and mortality.
The efficacy of the MA was evaluated at the end of the feeding test by measuring the following productive parameters: average weight gain (AWG) (g), average feed intake (AFI), feed conversion ratio (FCR) calculated by the relationship between AFI and AWG, during five weeks. Additionally, the weight and carcass yield were also determined following the methodology proposed by Magnoli et al.31. Each of these growth parameters was measured individually (per animal) and per treatment, and statistically analysed.
Biochemical parametersBlood samples (1ml/bird) were collected at the end of the feeding test. The serum was then obtained by centrifugation (2500×g for 15min at room temperature) and stored at −20°C for the determination of total proteins (TP), albumin (ALB). The total fraction of globulin (GLOB) was calculated subtracting the ALB from the TP, and the albumin:globulin ratio (ALB:GLOB) was determined. The enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT) and lactate dehydrogenase (LDH) were also determined using the colorimetric method with a commercial kit (Wiener Lab).
SlaughterAfter the fifth week, animals were slaughtered. Livers and intestines were collected. Liver weight was expressed as a percentage of the body weight (organ weight/100g live body weight).
Liver and intestine histopathologyPortions of approximately 6mm2 of liver and small intestine tissue samples (duodenum) were fixed in 4% (v/v) buffered-saline formaldehyde pH 7.2–7.4 at 4°C, dehydrated in a graded series of ethanol (30%, 50%, 70%, 80%, 90%, 95% and 100%) and xylene solutions, embedded in paraffin and cut in ±4μm histological serial-sections. The histological sections were stained with hematoxylin/eosin (H/E) for microscopic analysis. Liver slides were examined for typical intoxication signs and hepatocellular degeneration according to Magnoli et al.30. Intestines were examined for damage and inflammation using a standard histopathological grading system described by Del Carmen et al.16. High histological scores indicate increased damage in the intestines. Digital images were captured with an Axiophot microscope (Carl Zeiss, Thornwood, NY) fitted with a high-resolution Power shot G6 7.1megapixel digital camera (Canon INC, Japan). Digital image analysis and morphometric measurements were performed with Axiovision AxioVs40 V4.6.3.0 software (Carl Zeiss, Göttingen, Germany).
Statistical analysisData were analysed by a general linear mixed model (GLMM) (version 2.03; Córdoba, Argentina) and by analysis of variance (ANOVA). Means were compared using Fisher's protected least significant test (LSD) (p<0.05).
ResultsAverage weight gain, feed intake and feed conversion ratio determinationsThe weekly average weight gain (AWG) for each treatment is shown in Table 1. During week 5, the chickens that received only AFB1 (T2) significantly reduced the AWG (p˂0.0155), whereas those that received the toxin and MA (T3) and MA (T4) exhibited a significant increase in AWG during week 4 and week 5 (p≤0.0143 and p≤0.0155) with values similar to the control treatment (T1).
Effects of the mixed additive and aflatoxin B1 on the weekly average weight gain (AWG, g) and feed conversion rate (FCR, kg feed/kg body gain) of broilers.
| Treatments | Weight day 1 | Wk – 1 | Wk – 2 | Wk – 3 | Wk – 4 | Wk – 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AWG | FCR | AWG | FCR | AWG | FCR | AWG | FCR | AWG | FCR | ||
| T1 | 66.8a | 157.4b | 0.692 | 576.6b | 0.581 | 956.5b | 0.850 | 1259.7b | 1.177 | 1638.0c | 1.473 |
| T21 | 64.5a | 147.4a | 0.644 | 533.3a | 0.553 | 904.9a | 0.782 | 1169.7a | 1.107 | 1334.1a | 1.728 |
| T32 | 67.8a | 148.7a | 0.673 | 553.2a | 0.560 | 921.8ab | 0.799 | 1227.7b | 1.136 | 1351.9b | 1.732 |
| T4 | 69.4a | 159.2b | 0.685 | 567.7b | 0.590 | 940.8b | 0.864 | 1246.4b | 1.190 | 1622.9c | 1.487 |
| p-Value | 0.001 | 0.0095 | 0.033 | 0.0021 | 0.026 | 0.0094 | 0.017 | 0.0143 | 0.050 | 0.0155 | 0.050 |
T1: commercial diet; T2: AFB1 diet 1; T3: AFB1+mixed additive diet 2; T4: mixed additive diet.
Different letters within a column indicate significant difference according to the Fisher's least significant difference (LSD) test.
1AFB1 at 506000±22.1ng/kg.
20.1% mixing the lyophilized microorganisms (1:1): S. cerevisiae RC016 at 1×107cells/g and L. rhamnosus RC007 at 1×108cells/g.
Table 1 shows the results obtained for FCR. Treatments with the best FCR were those with toxins plus the MA (T3) and the mixed additive (T4), that is, a smaller amount of food was required for the production of a kilo of live weight, in comparison to the control (T1). In T2, the presence of AFB1 significantly reduced all the productive parameters tested (p<0.01).
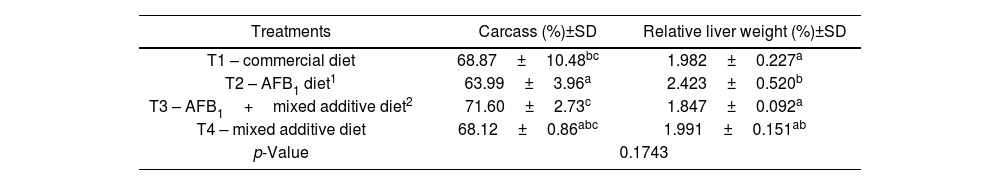
Table 2 shows the carcass performance for each of the treatments tested. The treatment with MA (T3) and the combination of the toxin with MA (T4) exhibited the highest values counteracting the harmful effect of mycotoxin. The presence of AFB1 (T2) significantly reduced the performance levels (p<0.1743).
Effect of the mixed additive and aflatoxin B1 on the carcass (%) of broilers and relative weight percentage (%) of broiler chicken livers in different treatments.
| Treatments | Carcass (%)±SD | Relative liver weight (%)±SD |
|---|---|---|
| T1 – commercial diet | 68.87±10.48bc | 1.982±0.227a |
| T2 – AFB1 diet1 | 63.99±3.96a | 2.423±0.520b |
| T3 – AFB1+mixed additive diet2 | 71.60±2.73c | 1.847±0.092a |
| T4 – mixed additive diet | 68.12±0.86abc | 1.991±0.151ab |
| p-Value | 0.1743 | |
The different letters in the columns indicate significant differences, according to Fisher's LSD test (p<0.0131). SD: standard deviation.
1AFB1 at 506000±22.1ng/kg.
20.1% mixing the lyophilized microorganisms (1:1): S. cerevisiae RC016 at 1×107cells/g and L. rhamnosus RC007 at 1×108cells/g.
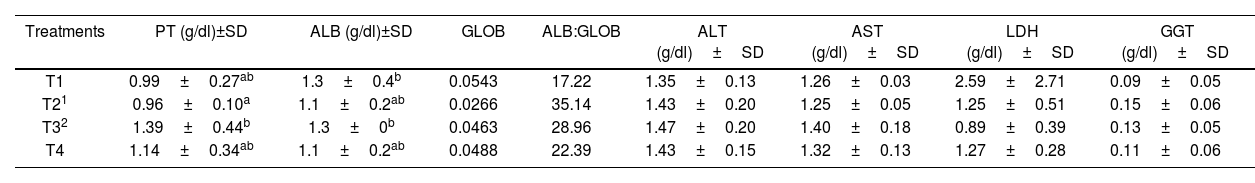
To assess the health status of the broilers fed with the different treatments, the blood test was collected the day before slaughter. The levels of PT, ALB, GLOB, ALB:GLOB, and AST, ALT, GGT, LDH enzymes were determined (Table 3). Total proteins and ALB, in general, were similar among all the tested treatments. However, GLOB levels were significantly reduced in the presence of AFB1 (T2). This effect was reflected in the relationship between ALB and GLOB, significantly increasing this coefficient. There were no significant differences among treatments for all the enzyme values tested.
Biochemical parameters from the serum of broiler chickens in the different treatments.
| Treatments | PT (g/dl)±SD | ALB (g/dl)±SD | GLOB | ALB:GLOB | ALT (g/dl)±SD | AST (g/dl)±SD | LDH (g/dl)±SD | GGT (g/dl)±SD |
|---|---|---|---|---|---|---|---|---|
| T1 | 0.99±0.27ab | 1.3±0.4b | 0.0543 | 17.22 | 1.35±0.13 | 1.26±0.03 | 2.59±2.71 | 0.09±0.05 |
| T21 | 0.96±0.10a | 1.1±0.2ab | 0.0266 | 35.14 | 1.43±0.20 | 1.25±0.05 | 1.25±0.51 | 0.15±0.06 |
| T32 | 1.39±0.44b | 1.3±0b | 0.0463 | 28.96 | 1.47±0.20 | 1.40±0.18 | 0.89±0.39 | 0.13±0.05 |
| T4 | 1.14±0.34ab | 1.1±0.2ab | 0.0488 | 22.39 | 1.43±0.15 | 1.32±0.13 | 1.27±0.28 | 0.11±0.06 |
T1: commercial diet; T2: AFB1 diet; T3: AFB1+mixed additive diet; T4: mixed additive diet. PT: total proteins; ALB: albumin; GLOB: globulin; ALB:GLOB: albumin:globulin ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; GGT: gamma-glutamyltransferase.
a,bDifferent letters within a column indicate significant difference according to the Fisher's least significant difference (LSD) test.
1AFB1 at 506000±22.1ng/kg.
20.1% mixing the lyophilized microorganisms (1:1): S. cerevisiae RC016 at 1×107cells/g and L. rhamnosus RC007 at 1×108cells/g.
Table 2 shows the relative weights of the livers from the different treatments. A significant increase (p<0.0131) was observed in the treatment with AFB1 (T2) compared to the other treatments. The addition of MA to the toxin diet decreased the toxic effect of AFB1 on the relative liver weight.
Figure 1 shows the macroscopic appearances of chicken livers that were fed different experimental diets. In treatment 1 (T1) a liver of small size, smooth surface, bright and intense wine-red colour corresponding to a normal liver was observed. In animals fed a diet contaminated with AFB1 (T2), a lighter colour, friable and pale in appearance was observed compared to controls (T1). The diets containing MA (T3 and T4), showed similar appearance and colour as the control treatment (T1), suggesting the preventive effect in the presence of the toxin.
Liver of broilers from the different treatment (T), T1 – commercial diet (CD); T2 – CD+AFB1 (506000±22.1ng/kg); T3 – CD+AFB1 (506000±22.1ng/kg)+0.1% mixed additive (MA), lyophilized microorganisms, the ratio of S. cerevisiae RC016 (1×107cells/g) to L. rhamnosus RC007 (1×108cells/g) was 1:1; T4 – CD+0.1% MA.
Figure 2 shows the photomicrographs of livers stained with hematoxylin and eosin. In T1 and T4, a slightly vacuolar (hydropic degeneration) appearance was observed in the peripheral lobular (hepatic) area. The T1 livers (control) showed a uniform pink tone throughout the body and bile duct hyperplasia. Hepatocytes (from four contiguous histopathological sections) showed an empty cytoplasm with basophilic and contracted nuclei of different diameters. The interlobular space showed two to three bile ducts in the portal space and bloodless vessels. A typical picture of chronic mycotoxicosis was considered here (T1). The livers from animals receiving the MA showed hepatocytes with slight hydropic degeneration (vacuolar), which is considered a normal microscopic appearance (T4). At the peripheral lobular interstitial level, two bile ducts and the interlobular vein were observed. The livers of chickens fed the AFB1 contaminated diet (T2) showed a slight microvacuolar fatty degeneration of hepatocytes. The coronal lobular section exhibited few sinusoidal spaces and little congestion. The cores were of regular size and shape. The livers of chickens fed the AFB1 contaminated diet and the MA (T3) had normal appearance and no proliferation of the bile ducts was observed. No signs of microvacuolar fat degeneration were found, suggesting the protective effect of the MA on hepatic aflatoxicosis.
Histopathology of the livers of broilers under different treatments (T), T1 – commercial diet (CD); T2 – CD+AFB1 (506000±22.1ng/kg); T3 – CD+AFB1 (506000±22.1ng/kg)+0.1% mixed additive (MA), lyophilized microorganisms, the ratio of S. cerevisiae RC016 (1×107cells/g) to L. rhamnosus RC007 (1×108cells/g) was 1:1; T4 – CD+0.1% MA. 40× magnification.
Figure 3 shows representative photomicrographs of the small intestine for each treatment at 2.5×, 10× and 40×. The T1 treatment exhibited grade 0, according to the denomination established by Del Carmen et al.16 without any evidence of epithelial alterations and the villi remained intact. Likewise, the chickens that only received the MA (T4) were similar to the control treatment. The presence of AFB1 (T2) showed alterations, thickening of the submucosa of the small intestine (grade 1) and proliferation of the Brünner glands (indicated by an arrow in Fig. 3 at 40×). In contrast, T3 (AFB1+MA) showed no histological damage in the small intestine, thus, MA reduced or prevented a possible intestinal injury induced by AFB1.
Representative micrographs of the small intestine of each treatment (T), T1 – commercial diet (CD); T2 – CD+AFB1 (506000±22.1ng/kg); T3 – CD+AFB1 (506000±22.1ng/kg)+0.1% mixed additive (MA), lyophilized microorganisms, the ratio of S. cerevisiae RC016 (1×107cells/g) to L. rhamnosus RC007 (1×108cells/g) was 1:1; T4 – CD+0.1% MA. Arrow indicates the presence of the Brünner glands.
Growth-promoting antibiotics are well documented to play an important role in the feed efficiency of poultry. Regulations and consumer demands decreased their use worldwide, resulting in increased research focused on the development of alternatives to maintain or improve poultry health and performance23,44.
S. cerevisiae RC016 and L. rhamnosus RC007 are industrially scalable strains that have been proven to possess probiotic and anti-mycotoxin properties; in addition, they were found to be GRAS for use as supplements in animal feed. They also demonstrated the ability to improve animal health and productivity when used in separate formulations22,25,40.
The final application of the MA in the present work was carried out in an in vivo test with broilers to assess its safety as well as its probiotic and anti-mycotoxin potential in a challenge with AFB1. In general, the tested productive parameters (AWG, FCR, and carcass performance) improved significantly in treatments with the MA. These results coincide with those of Bai et al.10 with L. fermentum and S. cerevisiae as probiotics at different doses (0.1–0.3%) and two growing periods (1–21 d and 22–42 d) achieving better results at 0.1% product in the initial phase diets compared to antimicrobial growth promoters.
Here, the treatment that combined MA with the AFB1 diet managed to counteract the decrease in weight gain, carcass performance, and histopathological damage caused by aflatoxicosis. Similar results to ours were obtained by Śliżewska et al.42 that tested the efficacy of a probiotic preparation containing Lactobacillus and S. cerevisiae species and also reversed the negative effects of treatments experimentally contaminated with AFB1. However, they tested two different doses (1 and 5mg/kg) and at a higher dose of AFB1, the probiotic could not avoid the histological changes induced by mycotoxin.
The determination of biochemical parameters allows to assess the nutritional status27. The liver has an essential role in the metabolism of nutrients, in the detoxification and excretion of hydrophobic and xenobiotic metabolites, in the synthesis of most plasma proteins, and in the synthesis, secretion, and conservation of bile acids that are essential for the intestinal absorption of fats and lipids, including fat-soluble vitamins. Therefore, the presence of liver disease is often recognized based on elevated serum activities of liver-derived enzymes such as AST, ALT, LDH and GGT28. In the present work, the broilers did not show any type of pathology or systemic effect that could be evidenced in the serum. The administration of probiotics in the present trial demonstrated their safety. Pizzolitto et al.38 agreed with our results demonstrating that animals fed with a diet containing 1.2mg AFB1/kg improved biochemical parameters through the application of S. cerevisiae CECT 1891 (5×109cells/l); although they added the yeast in the drinking water.
The liver is the main organ for detoxification and the main target organ of AFB133,34. In this study, the livers of chickens were examined for pathological changes. From the macroscopic examination, a beneficial effect resulting from the use of S. cerevisiae RC016 mixed with L. rhamnosus RC007 was observed, which was indicated by a slightly darker brown colour in the livers (Fig. 1, T3 and T4), suggesting a protective effect against aflatoxicosis. Pizzolito et al.38 demonstrated that S. cerevisiae CECT 1891 added to a diet (1010cells/kg) or to drinking water (109cells/l) had a beneficial protective effect on the liver histopathological changes of broilers fed AFB1 contaminated diets. It is important to highlight that in the present study, chickens received an AFB1 contaminated diet (506000ng/kg) that exceeded the recommended limit (20ng/kg)4 and the slight hydropic degeneration present in the control and MA treatments were probably due to this mycotoxin diet contamination. Histopathology of chicken livers with AFB1 showed bile duct proliferation and hepatocellular degeneration as a typical pattern of aflatoxicosis. The absence of microvacuolar fat degeneration suggested the protective effect of the MA in hepatic aflatoxicosis.
Upon ingestion of contaminated feed, the gastrointestinal tract (GIT) is particularly affected by mycotoxins. Generally, the intestinal barrier in the GIT functions as a filter against harmful mycotoxins. However, some mycotoxins have been found to exert detrimental effects on the GIT. For example, mycotoxins can alter normal intestinal functions such as barrier function and nutrient absorption. Some mycotoxins also affect the histomorphology of the intestine29. Akinrinmade et al.2 also demonstrated intestinal lesions induced by AFB1, but in rats. In the treatment with AFB1, the infiltration of leukocytes and lymphocytes was observed in the lamina of the intestinal mucosa. In the duodenum and ileum, exposure to AFB1 caused intestinal lesions such as the development of the subepithelial space and degeneration of the villi. Adverse effects in the intestine from exposure to AFB1 include disruption of the intestinal barrier, cell proliferation, cell apoptosis, and the immune system. In the present study, the Brünner glands that constitute mucin-secreting glandular acini located in the deep and submucosal mucosa layer of the duodenum, proliferated and probably secreted mucus, pepsinogen, and urogastrone in response to acid stimulation triggered by the presence of AFB1. Likewise, Del Carmen et al.16 found a significant decrease in intestinal damage through the use of a recombinant lactic acid bacteria treatment in a murine model of colitis, with similar findings as those in this study.
ConclusionThe addition of S. cerevisiae RC016 and L. rhamnosus RC007 into a MA in broilers diets yielded innovative and beneficial effects. On the one hand, it provided protection against mycotoxin contamination, by maintaining adequate levels of productive parameters, avoiding the deleterious effect of AFB1, but also exerting a beneficial influence as probiotic, contributing not only to increasing the productive parameters but also exerting a positive effect on health, evidenced through an improvement in biochemical and histopathological parameters. This is of great importance at the economic level for the avian production system.
FundingThis study was supported by grants from SECyT-UNRC331/16, PIP-CONICET GI11220120100156 and PICT3089/18.
Conflict of interestThe authors declare no conflict of interest.