Glyphosate-based herbicides (GBH) are the main pesticides applied worldwide on maize production. Glyphosate-resistant weeds led to the repeated application of high doses of the pesticide. In addition to environmental conditions, the presence of GBH affects the development of Aspergillus species and aflatoxin B1 (AFB1) production under in vitro conditions. The aim of this work was to evaluate the influence of a commercial GBH on growth and AFB1 production by Aspergillus flavus and Aspergillus parasiticus strains under different water activity (aW) conditions. The following concentrations of active ingredient glyphosate were evaluated: 20, 50, 200 and 500mM. The lag phase prior to growth and growth rate did not change at 20 and 50mM (that is, at field recommended doses) at 0.98 and 0.95 aW; however, at increasing GBH concentrations, between 200 and 500mM, the growth rate decreased at all aW conditions. In general, as the GBH concentration increased, AFB1 production decreased. However, a significant increase in toxin accumulation was found only at one of the aW conditions (0.95) at 21 days with 50mM of GBH in A. flavus and 20 and 50mM of GBH in A. parasiticus. These results show that, even though Aspergillus section Flavi growth did not increase, AFB1 production increased on maize grains at GBH concentrations similar to those of field recommended doses under favorable water availability and temperature conditions.
Los herbicidas a base de glifosato (HBG) son los más aplicados a nivel mundial en la producción de maíz. La aparición de malezas resistentes a glifosato condujo a la aplicación repetida de altas dosis. Además de las condiciones ambientales, la presencia de HBG afectan el desarrollo de especies de Aspergillus y la producción de aflatoxina B1 (AFB1). El objetivo de este trabajo fue evaluar la influencia de un HBG comercial en el crecimiento de cepas de Aspergillus flavus y Aspergillus parasiticus y su producción de AFB1 en granos de maíz, bajo diferentes condiciones de actividad de agua (aW). Se evaluaron las siguientes concentraciones de ingrediente activo glifosato: 20, 50, 200 y 500 mM. La fase de latencia y la velocidad de crecimiento no se modificaron con 20 y 50 mM (dosis recomendadas a campo) a 0,98 y 0,95 de aW; mientras que el crecimiento disminuyó en todas las condiciones de aW cuando las concentraciones de HBG fueron de 200 y 500 mM. En general, cuando aumentó la concentración de HBG, disminuyó la producción de AFB1. Hubo un aumento significativo en la acumulación de toxina solo en una condición de aW (0,95) a los 21 días con 50 mM de HBG en A. flavus, y con 20 y 50 mM en A. parasiticus. Estos resultados muestran que concentraciones de HBG similares a las dosis recomendadas para uso a campo no incrementan el crecimiento de Aspergillus sección Flavi en granos de maíz, pero sí la producción de AFB1, bajo condiciones favorables de disponibilidad de agua y temperatura.
Maize (Zea mays L.) is a crop grown throughout the world, with the United States, China, and Brazil being the top three maize-producing countries in the world, with a production of approximately 563 of the 717 million metric tons/year. Nowadays, Argentina ranks in the fourth position as maize producer and second as exporter. In the 2018/2019 season harvest, the total maize production reached 51 million metric tons, with a production record with respect to the previous season10.
Aflatoxins (AFs) are recognized as the mycotoxins with the most toxicological risks. They are related to agricultural crops infected by Aspergillus flavus and Aspergillus parasiticus species belonging to Aspergillus section Flavi43. They are known as Group 1 carcinogens because there is sufficient evidence of their carcinogenicity in humans27,31. AFs are reported worldwide in several agricultural crops, mainly maize, peanuts, pistachio nuts and cotton seeds19. The contamination of maize with AFs is a relevant factor, since this crop is an important constituent of food and feed around the world. Only for a few years mycotoxin contamination has been identified as a cause of concern in cereals and oilseed production30. The European Communities establish that maize before manufacturing and use as ingredient in foodstuffs, must not contain more than 10μg/kg of total AFs or 5μg/kg of aflatoxin B1 (AFB1)20, whereas the U.S. Food and Drug Administration determined that grains exceeding 20μg/kg of AFs cannot be exported and have to be destined only to livestock feed50. Therefore, maize producer countries are very much concerned about these AF regulated levels to enter the exportation market.
Previous studies have shown that Aspergillus section Flavi are present in high frequency in maize soils samples and maize grains6,12,33,35. Crop infection with aflatoxigenic species and subsequent AF production are produced in susceptible crop when environmental conditions are favorable. Warm and humid conditions as well as droughts predispose infection of the host crop with A. flavus. Many of the mycotoxigenic microfungi are weakly pathogenic and they benefit when stress weakens host resistance. Climate changes such as increased temperatures and decreased water availability provide an advantage to these pathogens36. There is a big concern with respect to the impact of these environmental conditions on A. flavus infection22,29. Under heat or drought stress conditions, an increase in the susceptibility of maize to aflatoxigenic species was recorded21. In this scenario, agronomic practices such as tillage and phytosanitary application also influence AF maize contamination34,38.
Glyphosate-based herbicides (GBHs) are of vital importance during the development of extensive crops for the control of weeds that compete with the crop and reduce yields. In reaction to the glyphosate-resistance problem, farmers have increased the dosage and frequency of GBH application5. Several GBHs are recommended by the United States Environmental Protection Agency (EPA) to control weeds in agricultural and non-agricultural environments49. In 2014, around 825000kg of GBHs were used worldwide on different agricultural crops47. These products are the most commercialized in the world, together with other herbicides such as atrazine or 2,4-D that control glyphosate-resistant weeds51. The extensive use of this herbicide has shown to cause a selection in microbial populations, with increases in populations of specific microbial taxa with capacity to tolerate the herbicide1,17,18. Previous studies have evaluated the effects of GBHs, in the development and mycotoxin production by Aspergillus section Flavi and Nigri only on culture media4,13–16,26. However, there is no information about the effects of these herbicides on aflatoxigenic fungi growth and AF production on natural substrates. Thus, the aims of the present study were to evaluate the effect of different concentrations of a commercial GBH on (i) the lag phase prior to growth, (ii) growth rate and (iii) AFB1 production by strains of Aspergillus section Flavi under different environmental conditions on maize grains.
Materials and methodsFungal strainsTwo aflatoxin- producing strains, A. flavus AFS 63 and A. parasiticus APS 55, were selected for this experiment. The strains were previously isolated from soil destined to maize crop in Argentina and their AF capacity was determined6. Both were identified based on morphological, physiological and molecular features according to Klich28, Pildain et al.37 and Samson et al.44,45. The nucleotide sequences for the β-tubulin and calmodulin gene of A. flavus AFS 63 (accession numbers: MH743102- MH743108) and A. parasiticus APS 55 (accession numbers: MH743103- MH743104) strains were deposited in the GenBank database. These strains were maintained by regular sub-culturing on 2% Malt Extract Agar (MEA) at 25°C until use, and at the same time kept on glycerol (15%) at −80°C in the culture collection at the Research Institute in Mycology and Mycotoxicology (IMICO-CONICET), National University of Río Cuarto, Córdoba, Argentina.
Substrate conditioningIrradiated maize grains (10–12kGy) (DK 7210, Dekalb, Monsanto, Buenos Aires, Argentina) with 98% germinative capacity were used. After the sterilization process, the grains were examined for sterility and absence of AFs, and then they were kept at 4°C until use3,48. Four hundred grams of irradiated maize grains were placed in sterilized glass flasks and re-hydrated with sterile distilled water to obtain water activity (aw) levels of 0.98, 0.95 and 0.932. The accuracy of aw modifications of the grains was confirmed at the beginning and during all the experiment with an AquaLab Series 3 (Decagon Devices, Inc., Pullman, WA, USA). The aW values assayed in this study were those most frequently reported during the different stages of the maize crop. Thus, at the early dough stage, the moisture content is about 40% (equivalent to 0.99 aW) without water stress effects; then the moisture content decreases to 30–35% at the mid-dough stage (equivalent to 0.95 aw) and to approximately 25% (equivalent to 0.93 aw) at full maturity over a period of about 4 a 6 weeks11.
GBH commercial formulation Roundup-Controlmax® (Monsanto, Buenos Aires, Argentina) with 720g/l of the active ingredient, a.i. glyphosate (N-phosphonomethyl glycine) was used in this study. A stock solution (2M) was prepared by dissolving 46.96g of the commercial formulation in 100ml of sterile distilled water (v/v), sterilized with 0.2μm filter (Microclar, Buenos Aires Argentina) and kept at 4°C until use. The appropriate volumes of this solution were applied and homogenized to the sterilized maize grains to obtain the required GBH final concentrations (20, 50, 200 and 500mM of active ingredient: glyphosate). The lower concentrations assayed belong to the GBH field recommended doses (20 and 50mM, equivalent to 2–2.5kg/ha)32. The higher levels (200 and 500mM) represent those resulting from accumulation in spill sites, especially in times of drought.
Inoculation and incubation conditionsThe grains were maintained at 4°C for 72h with manual periodic shaking to allow both aW and herbicide equilibrium. Twenty grams of maize grains were placed as a monolayer into sterile Petri dishes and inoculated centrally with 2μl of a spore suspension (1×106spores/ml) from a 7-day-old culture growing on MEA3. Briefly, the spores were collected using 10ml sterile water containing 0.05% Tween 80 and the surface of the colony was rubbed to harvest the spores. The spore suspension was decanted and counted using a hemocytometer (Boeco, Germany). The inoculated maize grain plates were enclosed in separate polyethylene bags according to the aW and placed in polyethylene plastic chambers accompanied by beakers (500ml) of glycerol/water solution of the same aW as the maize grains, to maintain the equilibrium relative humidity (ERH) in the chamber during incubation. Six replicates per treatment (0.98; 0.95; 0.93 aw levels and 20, 50, 200 and 500mM of GBH final concentrations) were included and incubated at 25°C for 21 days. The temperature used in this study is around the optimum for growth of Aspergillus sp.8 and represents the median typical temperature (20 and 30°C) for maize growing season until harvest. Control plates without herbicide were also prepared for each aW condition as controls.
Growth parametersAssessment of growth was done daily during the incubation period by the examination of maize grains with a binocular magnifier (10×) in each treatment. The diameter of each colony (in two directions at a 90° angle among them) was registered daily until the colony reached the edge of the plate. These data were used for the determination of lag phases prior to growth (hours, h) and growth rates (radius, mm/day). The data plots showed a linear trend between radius and time after the adaptation phase (lag phase prior to growth). Data was fitted using a linear model obtained by plotting the colony radius (mm) against time (days). The growth rate (mm/day) was calculated from the slope of the regression line for each strain, aW conditions and GBH concentration. Lag phases (h) were calculated by equaling the regression line formula to the original inoculum size (radius, mm)3. In each experiment the total number of growth analysis was 180, resulting from the three levels of aW×two strains×five GBH treatments×six replicates.
AFB1 extraction and quantificationAfter 7, 14 and 21 days of incubation, maize grains from each plate (controls and treatments) were removed and dried at 50°C for 24h, and stored at −20°C until analyzed. AFB1 were quantitatively determined by HPLC following the methodology proposed by Trucksess et al.48. Twenty grams of milled maize grains samples were homogenized with acetonitrile/water (90:10) and shaken in an orbital shaker and the extracts were filtered through filter paper (0.45μm, Microclar, Buenos Aires, Argentina). A 3ml aliquot of each extract was applied to a clean-up column (Mycosep 224 MFC, Romer). A 200μl aliquot was derivatized with 700μl of trifluoracetic acid/acetic acid/water (20:10:70). The derivatized AFB1 (50ml solution) was analyzed using a reversed-phase HPLC/fluorescence detection system. The HPLC system consisted of a Waters Alliance e2695 separations module, equipped with automatic injector, connected to a Waters 2475 Multi λ fluorescence detector. Chromatographic separations were performed on a stainless steel Supelcosil LC-ABZ C18 reversed-phase column (150×4.6mm i.d., 5μl particle size; Supelco, PA, USA). Fluorescence of AFB1 derivatives was recorded (λexc 330nm; λem 460nm) and the toxin was quantified by correlating the peak height of each extract with those obtained from Sigma Chemical (St Louis, MO, USA) standard curves. The detection limit (LOD) of the analytical method and the quantification limit (LOQ) were 2.2 and 11.0ng/g, respectively. The total number of AFB1 analysis was the same as that mentioned above for the growth assay.
Recovery assay of AFB1A stock solution (50μg/ml) of AFB1 (Sigma Chemical, St Louis, MO, USA) in methanol was prepared. Each AFB1-free finely ground maize grain sample (10g) was spiked with an equivalent of 0.5, 1.0 and 5μg of AFB1/g. Spiking was performed in triplicate and a single analysis of the blank sample was carried out. After leaving it for 18h to allow the solvent to evaporate, the extraction, detection and quantification were done using the protocol detailed above in the section “AFB1 extraction and quantification”.
Statistical analysisMean values are based on sextuplicated data. Data of growth were transformed to log10 (x+1) to achieve the homogeneity of variance. Means were compared by the Fisher's protected LSD test to determine the influence of aW, GBH concentration and strains on the lag phase and growth rate parameters. All toxin data were transformed to ln (x+1) for evaluating the significance among the means of the variables aW, GBH concentration, days and their interaction influence on the production of AFB1. The effect of GBH concentration, aW and days on AFB1 production was evaluated with a general and mixed linear model with heterogeneous variances for aW. The analysis was conducted using PROC GLM in SAS (SAS Institute, Cary, NC)40.
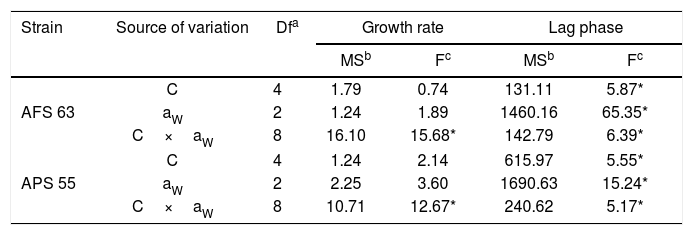
ResultsGBH effect on lag phase and growth rateStatistical analyses showed that two strains, aW and GBH concentration and their interaction significantly influenced (p<0.01) lag phase prior to growth, whereas in the growth rate only the interaction between aW and GBH concentration was statistically significant (Table 1).
Analysis of variance of effect of water activity (aW), concentration of GBH (C), and their interactions on lag phase prior to growth and growth rate of Aspergillus flavus (AFS 63) and Aspergillus parasiticus (APS 55) in maize grains.
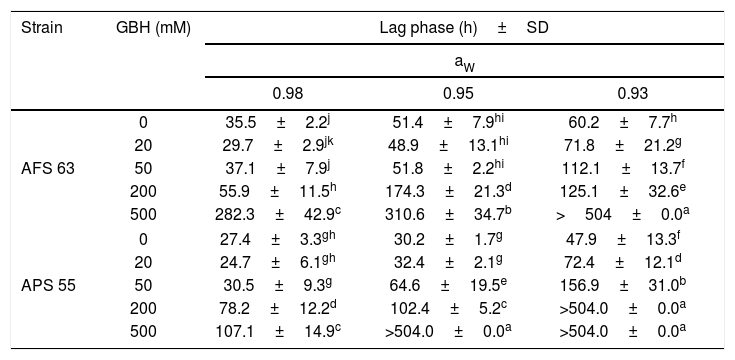
In general, in the lag phase prior to the growth analysis, in control treatments, as the aW was reduced in maize grains, this parameter increased significantly in both strains. This fact was more evident at the lowest aW tested; the lag phase was extended in 42 and 44% for AFS 63 and APS 55, respectively. With GBH, in general, the lag phase showed values similar to those in control at the field recommended doses (50 and 20mM of the herbicide) at 0.98 and 0.95 aW for AFS 63 and APS 55, respectively. At the highest concentrations (200 and 500mM) the lag phase of the strains was significantly extended, until exceeding the maximum incubation period (504h). This increase in the length of the lag phase was more evident at 0.93 aW even with 20mM of GBH (p<0.05) (Table 2).
Effect of GBH on lag phase (h) of Aspergillus flavus (AFS 63) and Aspergillus parasiticus (APS 55) on maize grains at different water activity (aW) levels.
| Strain | GBH (mM) | Lag phase (h)±SD | ||
|---|---|---|---|---|
| aW | ||||
| 0.98 | 0.95 | 0.93 | ||
| AFS 63 | 0 | 35.5±2.2j | 51.4±7.9hi | 60.2±7.7h |
| 20 | 29.7±2.9jk | 48.9±13.1hi | 71.8±21.2g | |
| 50 | 37.1±7.9j | 51.8±2.2hi | 112.1±13.7f | |
| 200 | 55.9±11.5h | 174.3±21.3d | 125.1±32.6e | |
| 500 | 282.3±42.9c | 310.6±34.7b | >504±0.0a | |
| APS 55 | 0 | 27.4±3.3gh | 30.2±1.7g | 47.9±13.3f |
| 20 | 24.7±6.1gh | 32.4±2.1g | 72.4±12.1d | |
| 50 | 30.5±9.3g | 64.6±19.5e | 156.9±31.0b | |
| 200 | 78.2±12.2d | 102.4±5.2c | >504.0±0.0a | |
| 500 | 107.1±14.9c | >504.0±0.0a | >504.0±0.0a | |
Mean values are based on sextuplicated data.
Mean values in each strain with a letter in common are not significantly different according to the LSD test (p<0.05). >504.0: under these conditions, the strains were not able to reach the exponential phase. SD: standard deviation.
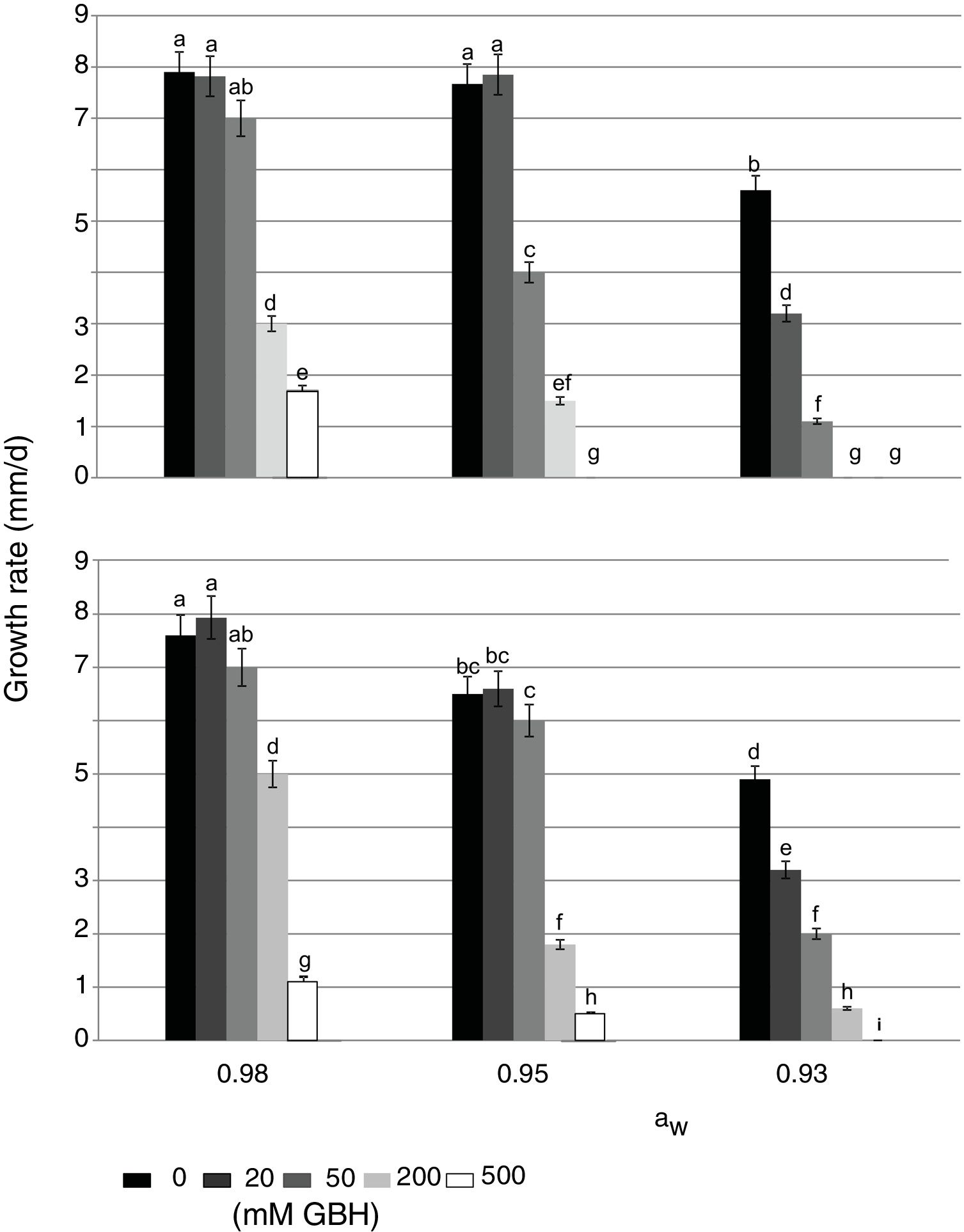
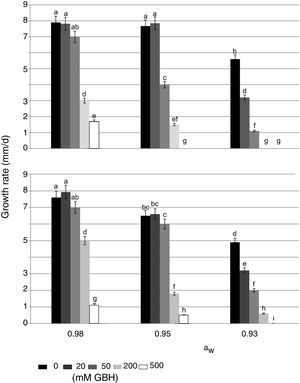
In control treatments, similarly to what occurred with the lag phase, in general the growth rate of both strains decreased when aW on maize grains was also reduced. Optimal condition for growth was 0.98 aW for the AFS 63 strain and 0.98 and 0.95 aW for the AP 55 strain. In herbicide treatments, a significant reduction in this parameter was also observed when the amounts of GBH increased. In general, both strains showed a similar growth profile in the presence of the herbicide, this being more evident at the highest aW condition (Fig. 1). At 0.98 and 0.95 aW with 20 and 50mM of GBH the growth rate of both strains was similar to the respective controls, except in the APS 55 strain with 50mM at 0.95 aW, where a significant reduction with respect to the control was registered (Fig. 1B). From 200mM GBH, a statistically significant decrease in the growth rate of both strains was observed (p<0.05). At the lowest aW condition there was an inversely proportional relationship between the growth rate and the GBH increase in the substrate. The development of AFS 63 strain was completely inhibited only at 500mM (Fig. 1A), whereas the growth of the APS 55 strain was inhibited from 200mM (Fig. 1B).
Effect of GBH (mM) on growth rate of Aspergillus flavus AFS 63 (A) and Aspergillus parasiticus APS 55 (B) on maize grains under different water activity (aW) levels. Mean values with a letter in common are not significantly different according to the LSD test (p<0.05). Mean values are based on sextuplicated data.
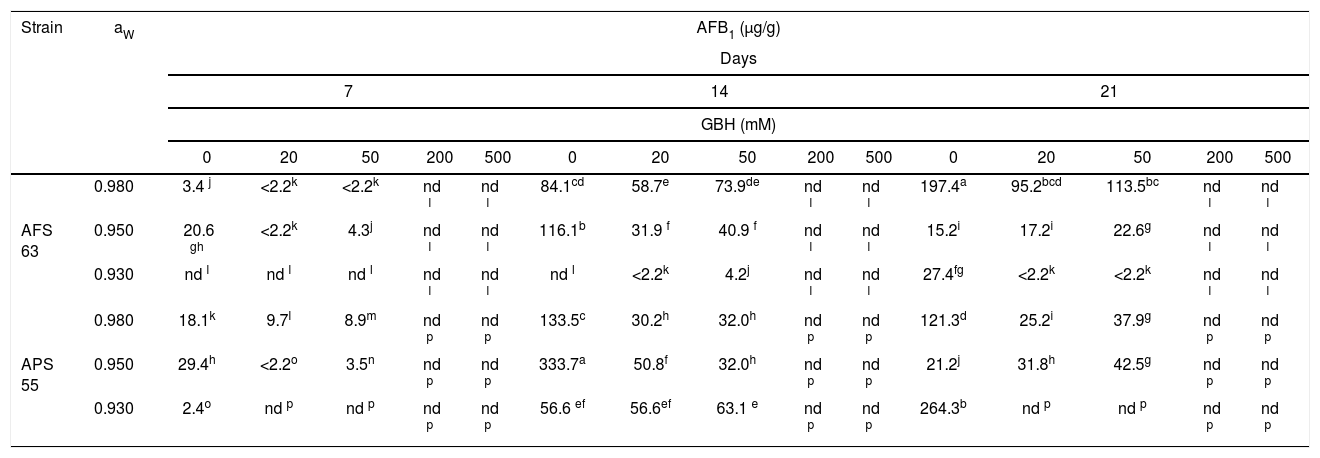
Table 3 shows the AFB1 accumulation on maize grains that were added several amounts of GBH and conditioned at three aW levels and 25°C. In most of the development conditions, for the APS 55 strain, AFB1 production was greater than the AFB1 accumulation observed for AFS 63. In control treatments, the highest accumulation of AFB1 was registered for the AFS 63 strain at 21 and 14 days of incubation at 0.98 and 0.95 aW; while for the APS 55 strain, this fact was recorded at 14 and 21 days at 0.95 and 0.93aW, respectively. In general, as the GBH concentration was increased, toxin production was inhibited. However, a significant increase in toxin accumulation was found only in one aW condition (0.95) at 21 days of incubation with 50mM of GBH for AFS 63 and with 20 and 50mM of GBH for the APS 55 strain (p<0.05). From 200mM of the herbicide, the strains were not able to produce AFB1 under any of the conditions assayed. Likewise, 0.93 aw was the condition that most inhibited the production of AFB1 regardless of the concentration of GBH used.
Effect of GBH on AFB1 production by Aspergillus flavus AFS 63 and Aspergillus parasiticus APS 55 on maize grains under different water activities (aW).
| Strain | aW | AFB1 (μg/g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | ||||||||||||||||
| 7 | 14 | 21 | ||||||||||||||
| GBH (mM) | ||||||||||||||||
| 0 | 20 | 50 | 200 | 500 | 0 | 20 | 50 | 200 | 500 | 0 | 20 | 50 | 200 | 500 | ||
| AFS 63 | 0.980 | 3.4 j | <2.2k | <2.2k | nd l | nd l | 84.1cd | 58.7e | 73.9de | nd l | nd l | 197.4a | 95.2bcd | 113.5bc | nd l | nd l |
| 0.950 | 20.6 gh | <2.2k | 4.3j | nd l | nd l | 116.1b | 31.9 f | 40.9 f | nd l | nd l | 15.2i | 17.2i | 22.6g | nd l | nd l | |
| 0.930 | nd l | nd l | nd l | nd l | nd l | nd l | <2.2k | 4.2j | nd l | nd l | 27.4fg | <2.2k | <2.2k | nd l | nd l | |
| APS 55 | 0.980 | 18.1k | 9.7l | 8.9m | nd p | nd p | 133.5c | 30.2h | 32.0h | nd p | nd p | 121.3d | 25.2i | 37.9g | nd p | nd p |
| 0.950 | 29.4h | <2.2o | 3.5n | nd p | nd p | 333.7a | 50.8f | 32.0h | nd p | nd p | 21.2j | 31.8h | 42.5g | nd p | nd p | |
| 0.930 | 2.4o | nd p | nd p | nd p | nd p | 56.6 ef | 56.6ef | 63.1 e | nd p | nd p | 264.3b | nd p | nd p | nd p | nd p | |
Mean values are based on sextuplicated data.
Standard error: 9.82366914.
Values with a letter in common are not significantly different according to the LSD test (p<0.05). nd: not detected. LOD: 2.2ng/g.
LOQ: 11.0ng/g.
This study showed that all the variables analyzed affected the acclimation period (lag phase prior to growth) of A. flavus and A. parasiticus strains, while the mycelial growth rate was affected by GBH concentration, aW and their interactions. Both strains showed a similar behavior on control treatments, the aW near to optimal growth produced an increase in the fungal growth rate and a decrease in the acclimation period. Similar results were informed by Bernáldez et al.8 and Giorni et al.25, who reported that aW levels of 0.99 and 0.95 were the best conditions for growth at 25 and 30°C of A. flavus strains on maize-based media and maize. In treatments supplied at low concentrations of GBH (20 and 50mM) at optimal aW conditions (0.98 and 0.95) for development, both the growth rate and lag phase showed similar values to the ones in the controls. Only the growth rate of one of the strains (AP 55) was negatively affected by 50mM of GBH at 0.95 aW. In general, these results indicate that the presence of the herbicide at field recommended doses does not stimulate the development of these strains. Contrarily, the development was affected significantly at the highest concentrations of GBH at all aW tested. This fact indicates that the herbicide has inhibitory effects when the concentrations are similar in spill situations. Despite the intense use of GBH in maize production, there is no information about their influence on development and AF production by Aspergillus section Flavi on natural substrates such as maize grains. Some authors established the negative effect of other pesticides on the growth of Aspergillus species on different cereal grains. Reddy et al.42 evaluated the efficacy of fungicides on control growth of Aspergillus spp. in rice. They showed that lower or similar doses than the ones used in the present work (5mM of carbadazyme and 20mM of triziclazole) totally inhibited fungal growth. Although GBHs are not used as a fungicide agent, the Aspergillus growth inhibition observed at doses higher than those recommended mainly at limiting conditions of water availability agrees with the growth inhibition produced by fungicides. Other authors obtained different results to those registered in this study, observing a significant decrease in growth of Aspergillus section Flavi strains when they developed on water agar synthetic media and YES medium with GBH levels (0.5 to 5mM and 0.5 to 10.0mM, respectively) lower than those used in this study26,41. The differences observed with these studies could be explained by the high nutritional value of the substrate used (maize grains) that could improve glyphosate tolerance.
With regard to toxin production, the results showed an inhibition in AFB1 production as GBH concentration increased. However, the increase in toxin accumulation at recommended doses (20 or 50mM of GBH depending of the strains) is noticeable at 0.95 aW and 21 days of incubation. The addition of herbicide concentrations that can be found in spill sites (200 and 500mM) caused a reduction in growth rate and also inhibited AFB1 production under any conditions tested. These results indicate that under certain aW conditions, even though the growth does not increase with respect to control, toxin production is stimulated. Reddy et al.42 also informed that AFB1 production by A. flavus decreased while the concentration of the fungicides carbadazyme and trizicazole in rice increased. In addition, they observed a total inhibition of toxin production at a lower concentration (5mM of carbadazyme and 20mM of tricicazole) than the ones observed in the present study. In a previous work, Benito et al.7 also observed a significant increase in growth rate and AFB1 production as the concentration of atrazine (other herbicide applied together with GBH) in maize extract agar increased from 5 to 100mM.
Some authors, such as Barberis et al.4 evaluated the effect of lower doses of GBH on growth and AFB1 production by Aspergillus section Flavi on maize-based medium. They found that growth increased significantly from 0.5 to 10mM of herbicide, being this increase more evident at 5 and 10mM. Concerning AFB1 production, no significant differences were shown between the different GBH concentrations and aW assayed. Our results partially agree with those obtained by these authors, because the increase in AFB1 production was observed only at 0.95 aW, but at the highest doses of GBH (20 and 50mM).
Glyphosate-resistant crops (GRCs) have been developed for soybean, maize, cotton, among others. These GRCs have quickly gained attention due to their resistance to GBHs which simply allow farmers to apply GBHs for a broader range of applications. However, due to the development of GRCs, the use of glyphosate has also increased since its introduction. In addition, it is expected that crops such as soybean and corn may contain higher amount of glyphosate residues. Glyphosate residues increased with repeated applications to the crop, maize sprayed at full bloom of the plant contained about 5–10 times more glyphosate than plants sprayed only early in the growing season. The current maximum residue levels of glyphosate in maize grains are 5mg/kg for FAO/WHO and US-EPA respectively53. A survey of maize and soy products collected from Philadelphia and the U.S. metropolitan area showed that ten out of twenty-eight (36%) samples contained glyphosate at a concentration above the limits of the techniques used for detection9. The mechanisms of glyphosate resistance identified in fungi and bacteria are the same as those described in plants. The biocidal activity of glyphosate is associated with the inhibition of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS). Glyphosate thus stops the sixth step in the shikimate pathway (conversion from shikimate-3-phosphate to EPSP), which is required for the production of aromatic amino acids and secondary compounds with defense functions in plants and many microorganisms23,46,52. Some fungal soil species can express glyphosate sensitive enzymes (class I EPSPS), and several of them express GP-tolerant forms (class II EPSPS) and are not inhibited or are even stimulated when crops are treated with GBH24,39. However, there is no information about the effect of these herbicides on AFs synthesis.
The data of the present work showed that A. flavus and A. parasiticus can develop and produce AFB1 in the presence of GBH concentrations similar to the field recommended doses under water availability of 0.98 and 0.95.
ConclusionThis work showed the importance of avoiding repeated herbicide applications due to the possible stimulation that they produce in these fungal species, at doses of 20 and 50mM. This indirect effect of glyphosate needs to be taken into account by regulatory agencies in the implementation of good agricultural practices to prevent the development of these species and subsequent AFB1 production; avoid economic losses, changes on the organoleptic characteristics of the grain, and risks in human and animal health.
FundingThis work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-PICT-0943/14) and Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECYT-UNRC-18/453).
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-PICT- 0943/14) and Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECYT-UNRC- 18/453).