The aim of this study was to determine the impact of Kluyveromyces marxianus VM004 culture conditions on the cell wall (CW) structure and its influence on aflatoxin B1 binding. The yeast was inoculated into two types of culture media: yeast extract-peptone-dextrose (YPD) broth and dried distiller's grains with solubles (DDG). The CW was extracted from the biomass produced in these media. AFB1 (150ng/ml) adsorption tests using the biomass (1×107cells/ml) and the CW (0.001g) were performed at pH 2 and pH 8. Transmission electron microscopy (TEM) evaluated the CW thickness, and infrared spectroscopy (IR) determined the CW composition. Biomass production in YPD was higher than that in DDG. Cell diameter (μm) and CW thickness (μm) increased in the DDG medium. The CW percentage obtained in DDG was higher than that in YPD. The absorbance of carbohydrates by IR was higher in YPD. pH influenced AFB1 adsorption, which was lower at pH 8. The proportion of β-glucan and chitin present in CW was higher in the YPD medium. The IR method allowed to study the CW carbohydrate variation under the influence of these carbon sources. In conclusion, the culture media composition influenced the β-glucan and chitin composition and consequently, AFB1 adsorption.
El objetivo de este estudio fue determinar el impacto de las condiciones de cultivo de Kluyveromyces marxianus VM004 en la estructura de la pared celular (PC) y su influencia en la adsorción de la aflatoxina B1 (AFB1). La levadura fue inoculada en dos medios de cultivo, extracto de levadura-peptona-dextrosa (YPD) y granos de destilería secos con solubles (DDG). De la biomasa obtenida en estos medios se extrajo la PC. Los ensayos de adsorción de la AFB1 (150ngml−1) utilizando la biomasa (1×107celml−1) y la PC (0.001g) se realizaron con pH 2 y pH 8. El espesor y la composición de la PC se determinaron por microscopía electrónica de transmisión (TEM) y espectroscopía infrarroja (IR), respectivamente. La producción de biomasa en YPD fue superior a la lograda en DDG. El diámetro de las células (μm) y el espesor de la PC (μm) aumentaron en el medio DDG. El porcentaje de PC obtenido en DDG fue superior al obtenido en YPD. La absorbancia de carbohidratos por IR fue mayor en YPD. El pH influyó en la adsorción de la AFB1, que fue menor al pH 8. La relación de β-glucano/quitina presente en la PC fue mayor en medio YPD. El método de espectroscopía IR permitió estudiar la variación de carbohidratos en la PC bajo la influencia de estas fuentes de carbono. En conclusión, la composición de los medios de cultivo incidió en la relación de β-glucano y quitina y, en consecuencia, en la adsorción de la AFB1.
In recent years, the so-called non-conventional yeast species have acquired relevance because of their positive contribution to food and beverages. Kluyveromyces species participate in the fermentation of beverages, dairy products, bakery products, sausages and various vegetables11,29. Among them, Kluyveromyces lactis and Kluyveromyces marxianus are considered generally recognized as safe (GRAS) microorganisms in the United States and have qualified presumption of safety (QPS) status in the European Union16. K. marxianus can grow on a variety of substrates and is the major producer of industrial enzymes for fermented foods and beverages, biofuels, and cell factory applications30,32. They are also a source of oligonucleotides, flavor enhancers in food products, prebiotics and immunostimulators11. In addition, they have been recognized as probiotic microorganisms4,5. Probiotics are defined as ‘live microorganisms which, when administered in adequate amounts, exert a beneficial effect on the health of the consumer7. In the last years, functional foods that contain probiotic microbial strains responsible for health benefits in the host are being designed6,11.
Moreover, mycotoxins are natural products, toxic secondary metabolites produced by filamentous fungi. The most important mycotoxins are aflatoxins (AFs), mainly represented by aflatoxin B1 (AFB1). Aflatoxins are produced by Aspergillus section Flavi group species. Economically and biologically the most important fungal species able to produce AFs are Aspergillus flavus and Aspergillus parasiticus1,10. Aflatoxins are among one of the most significant hazards to the feed supply chain and pose a threat to feed industries worldwide with a direct impact on feed safety, animal health and productivity. The occurrence of AFs is common in feed as natural contaminants in a variety of agricultural commodities of plant origin, especially in cereal grains, and are therefore often detected in animal feeds containing corn, soybean, and wheat, but can also be present in silage, haylage and pasture33. The ingestion of mycotoxin contaminated feeds can cause both acute and chronic diseases known as mycotoxicoses in most animal species such as pigs, dogs, cats, rainbow trouts, and ducklings17.
Poor harvesting practices, improper drying, handling, packaging, storage, and transport conditions of cereals contribute to fungal growth and increase the risk of mycotoxin production. One of the strategies to prevent mycotoxicoses is the dietary supplementation with substances to make the toxin not available in the digestive tract and, therefore, to reduce its adverse effect. These strategies include the use of yeast cell wall (YCW) with potential to adsorb mycotoxins23,34.
The YCW determines the shape of the cell and the integrity of the organism during cell growth and division. Its composition can vary under different growth conditions including type of culture, carbon source, temperature, pH, and aeration19. In particular, agroindustrial wastes, rich in carbohydrates and other nutrients, represent a good choice for microbial biomass biosynthesis28. K. marxianus was used in several biotechnological applications; however, no studies have shown the influence of the culture medium on the composition of the cell wall (CW) and its relationship with the adsorption of mycotoxins. Therefore, the aim of this study was to determine the impact of K. marxianus VM004 culture conditions on the structure of CW and its effect on AFB1 binding.
Materials and methodsMicroorganism and culture mediaK. marxianus VM004 was isolated from cheese whey, and characterized in our laboratory (and selected for its probiotic properties which were previously tested in in vitro and in vivo studies)4,14.
K. marxianus VM004 was identified by molecular techniques through DNA extraction and sequencing of ITS regions: ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), comparing sequences with the basic local alignment search tool (BLAST) within the National Center for Biotechnology Information (NCBI) database4. The strain was deposited in the National University of Rio Cuarto, Cordoba, Argentina (RC) collection center. The obtained sequences were deposited in GenBank under accession number KY421190 (see http://www.ncbi.nlm.nih.gov/nucleotide).
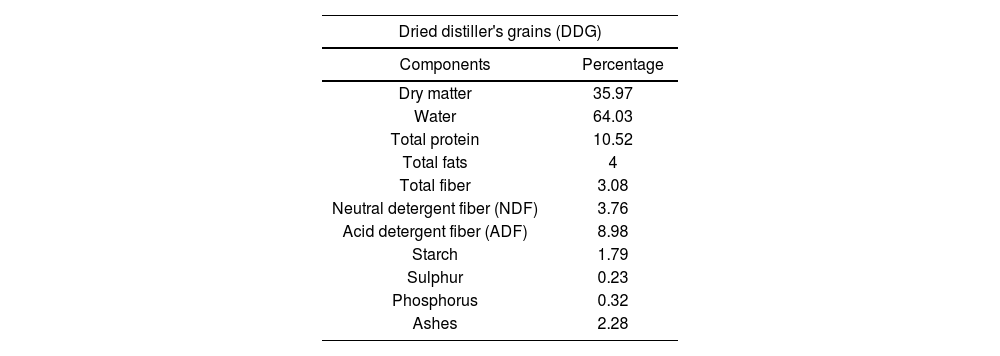
For the yeast growth, two culture media were used: yeast extract-peptone-dextrose broth (YPD) and dried distiller's grains with solubles (DDG). YPD medium was prepared according to the manufacturer's instructions. DDG was obtained from a local bioethanol producing plant. DDG medium was prepared by adding water (300ml) to 75g DDG, left at fluent steam for 20min and then filtered. Then, yeast extract (1g) and 0.1g K3PO4 were added to 100ml filtered DDG and autoclaved at 121°C for 15min. Its composition is shown in Table 1. The estimation of reducing sugars from the DDG medium was determined using the dinitrosalicylic acid (DNS) method. The amount of glucose from the DDG medium was 2.67g/l while the amount of glucose in YPD was 20g/l.
Dried distiller's grains (DDG) centesimal composition.
| Dried distiller's grains (DDG) | |
|---|---|
| Components | Percentage |
| Dry matter | 35.97 |
| Water | 64.03 |
| Total protein | 10.52 |
| Total fats | 4 |
| Total fiber | 3.08 |
| Neutral detergent fiber (NDF) | 3.76 |
| Acid detergent fiber (ADF) | 8.98 |
| Starch | 1.79 |
| Sulphur | 0.23 |
| Phosphorus | 0.32 |
| Ashes | 2.28 |
Yeast biomass production was performed following the methodology proposed by Nguyen et al. with some modifications20. The colony grown on malt extract agar (MEA) was inoculated into an Erlenmeyer flask (of 50ml) containing YPD broth and incubated at 28°C for 12h to serve as a pre-inoculum. From this pre-inoculum, a cell suspension (1×107cells/ml) was performed, and inoculated into five 500ml Erlenmeyer flasks with 200ml of medium each (total volume 1000ml) and incubated at 28°C for 12–14h with aeration (150rpm). Yeast cells were cultured to late exponential phase (about 12h). They were harvested by centrifugation at 5000rpm for 10min, washed three times with water and twice with cold (4°C) 0.1M Na2PO4 buffer at pH 8.5. The biomass obtained from the YPD and DDG media was dried in forced air oven until constant weight and, the weight was expressed as g biomass/l culture medium. Means and standard error (SE) were compared using the Fisher's protected least significant test (LSD) (p<0.0001). The analysis was conducted using Info Stat (version 2.03; University of Cordoba, Argentina) software.
Yeast cell wall preparationYeast cells used for the CW preparation were suspended in 0.1M Na2PO4 buffer pH 8.5, and an equal volume of glass beads (0.45mm diameter). They were cooled to 4°C and broken by mechanical shaking for 30s. The homogenate was cooled and disrupted for 30s and this procedure was repeated five times. The glass beads were removed from the homogenate by decanting, and the CW was harvested by centrifugation at 5000rpm for 15min. The CW was washed five times with 0.1M Na2PO4 buffer pH 8.5 and then, washed four times with distilled water. The temperature was kept below 4°C during all operations. The CW was dried in forced air oven until constant weight.
Ultrastructural analysis of yeast cellsYeast cell samples from the two different culture media were homogenized for 30min, and centrifuged for 10min at 10000rpm. The dry pellet was processed for TEM. The samples were processed following the methodology described in Pereyra et al.25. The sections were examined in an Elmiskop 101 transmission electron microscope (Siemens, Germany).
Infrared spectroscopyThe variation in the composition of the CW under the influence of the different culture media (YPD and DDG) was determined by the Fourier transformed infrared spectroscopy methodology. Dried CW (1mg), mixed with potassium chloride (KCl) (200mg), ground in an agate mortar and then, a tablet was made under pressure (≈15toncm−1) applying dynamic vacuum for 15min.
Measurements were performed in a Nicolet FTIR Impact 400 spectrometer. The spectrum includes an accumulation of 200 measurements to increase signal/noise ratio, and measured between 4000 and 400/cm with a resolution of 4/cm.
Aflatoxin B1 determinationsAflatoxin B1 production, detection and quantificationAflatoxins for in vitro assays were produced via the fermentation of rice by A. parasiticus NRRL 299913 (USDA, Agricultural Research Service, Peoria, IL). The fungus was grown on malt extract agar (MEA). AFs were extracted with chloroform following the procedure described in AOAC3.
The sterile substrate (20g) was placed in Erlenmeyer flasks and inoculated with 2ml of an aqueous suspension containing 106spores/ml. Cultures were allowed to grow for 7 days at 25°C in the darkness and were shaken daily. On day seven, the Erlenmeyer flasks were autoclaved and the culture substrate was dried for 48h at 40°C in a forced air oven and then ground to a fine powder. The total AFs content in the culture extract was determined by high performance liquid chromatography (HPLC)31. The HPLC instrument was a Hewlett Packard chromatograph with a loop of 20ml, equipped with a spectrofluorescence detector and a C18 column (Supelcosil LC-ABZ, Supelco; 150mm, 4.6mm, 5mm particle size) connected to a precolumn (Supelguard LC-ABZ, Supelco; 20mm, 4.6mm, 5mm particle size). AFB1 was the only one produced in the highest concentration (60μg/g). Levels of AFG1, AFB2 and AFG2 were detectable but not quantifiable.
Aflatoxin B1 adsorptionAfter the previously described incubation time, 1ml 107cells/ml or 0.001g YCW was added to a microtube. The suspension was centrifuged to obtain a pellet and the supernatant was discarded. Cells or CW were washed with distilled water to remove traces of YPD or DDG broth. A pH 2 solution (1ml) (containing 50ml of potassium chloride 0.2M and 13ml of hydrochloric acid 0.2M) and a pH 8 solution (containing 100ml of 0.1M KH2PO4 and 93.4ml of 0.1M NaOH) containing 150ng/ml AFB1 were added to each microtube containing the pellet. The mixture of yeast cells or CW and AFB1 was homogenized and incubated on a rotary agitator at 37°C for 40min at 100rpm. After the incubation period, the mixture suspensions were centrifuged and the supernatant containing the free toxin was transferred to another microtube. Controls only with AFB1 and at each pH were obtained separately and performed in duplicates. The dose selected for AFB1 was a proven one to reduce the productive performance in production animals18.
ResultsBiomass productionThere were no significant differences in the biomass production of K. marxianus grown in YPD broth (4.98g/l) and in DDG broth (4.24g/l) (p<0.0001).
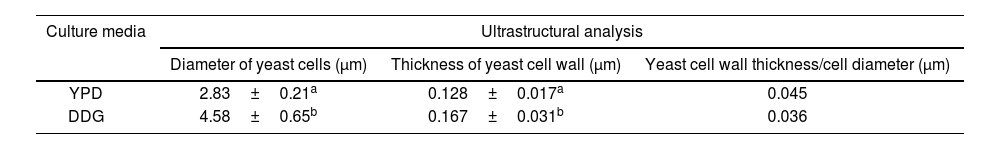
Ultrastructural analysis of yeast cells after different cultural conditions by transmission electron microscopyTable 2 shows the mean values of the yeast cell diameter and CW thickness for K. marxianus for each culture medium studied. The number of measurements for yeast cell diameter and thickness were 10 and 30, respectively. The yeast cell diameter and CW thickness of K. marxianus increased by 61.84% and 30%, respectively using DDG. The CW thickness/yeast cell diameter (μm) relationship was 25% higher using YPD.
Ultrastructural analysis of Kluyveromyces marxianus VM004: relationship between cell wall (CW) thickness and yeast cell diameter (μm).
| Culture media | Ultrastructural analysis | ||
|---|---|---|---|
| Diameter of yeast cells (μm) | Thickness of yeast cell wall (μm) | Yeast cell wall thickness/cell diameter (μm) | |
| YPD | 2.83±0.21a | 0.128±0.017a | 0.045 |
| DDG | 4.58±0.65b | 0.167±0.031b | 0.036 |
YPD: yeast extract-peptone-dextrose broth; DDG: dried distiller's grains with solubles. The same letters do not indicate significant differences. Statistical analyses were performed for each column separately according to Fisher's least significant difference test (LSD) (p<0.0001).
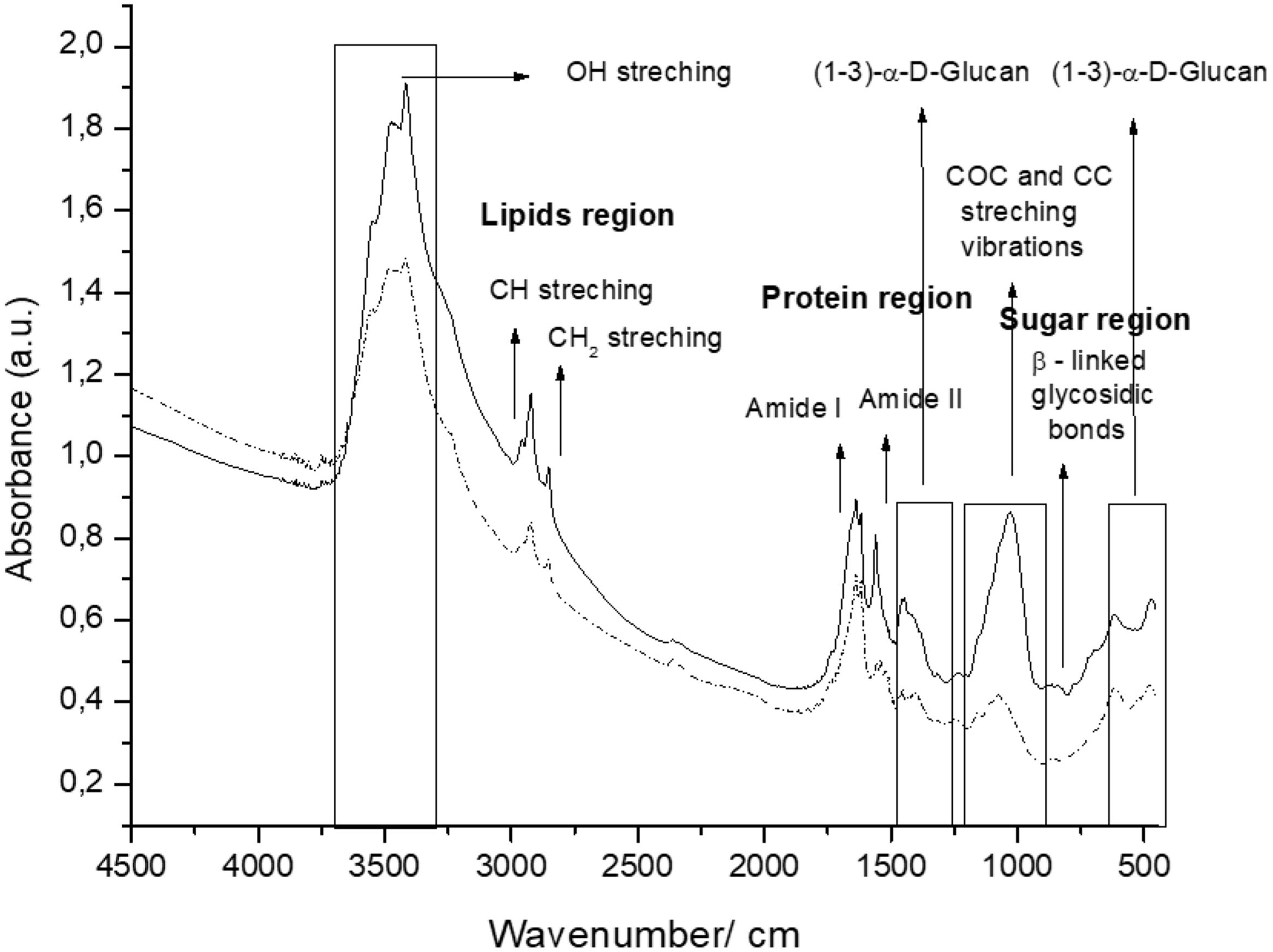
The spectra of the CW coming from the two culture media tested included three (3) regions: polysaccharides (950–1185/cm, proteins (1480–1700/cm) and lipids (2840–3000/cm) (Fig. 1).
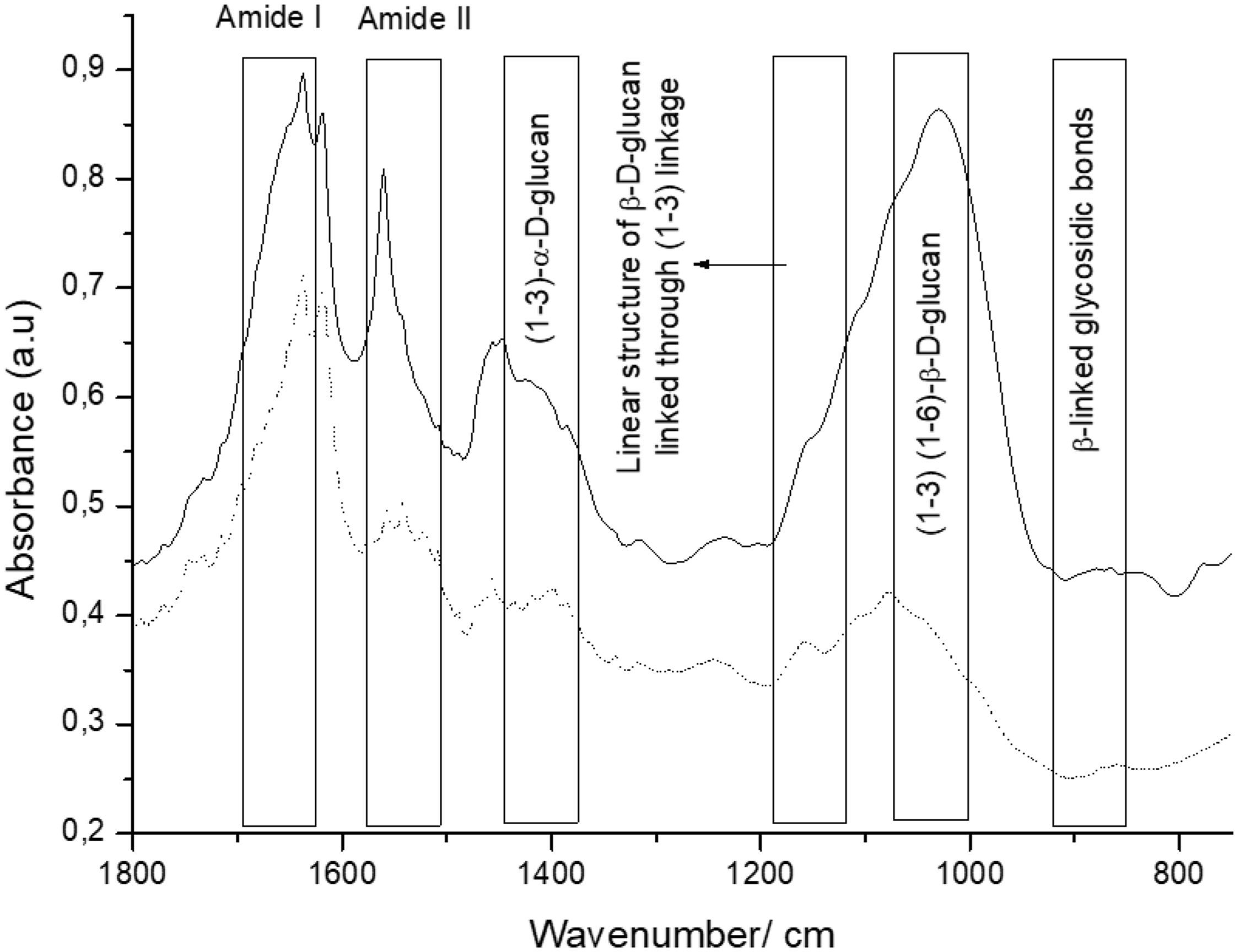
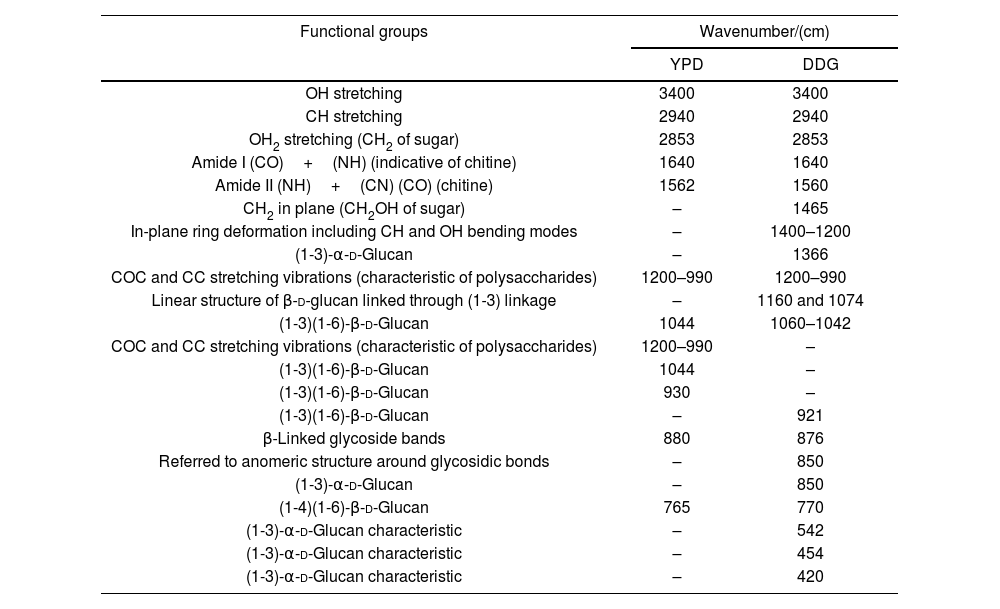
β-Glucans and chitin present in the CW are the chemical structures implicated in the AFB1 adsorption process. Figure 2 shows the IR spectra of β-glucans and amide bands from K. marxianus VM004 cultures in both culture media tested. The spectrum of YCW in YPD shows bands corresponding to OH stretching and CH stretching in the region between 2900 and 3500/cm. Furthermore, the infrared bands assigned to amide I and amide II (typical of chitin) were present at 1640 and 1562/cm, respectively. Vibration between C and O was identified in the region between 1000 and 1200/cm. β-Linked glycosidic bands were appreciable at 880/cm. In addition, in this case, (1-4)(1-6)-β-glucan absorption band was assigned at 765/cm. With regard to the spectrum of YCW taken in the DDG medium, similar peaks to those observed in YPD for OH and CH stretching were found at higher wavenumbers, being ∼3400/cm and 2940; 2853/cm, respectively. Moreover, the infrared bands assigned to amide I and amide II (typical of chitin) were present at 1640 and 1560/cm, respectively. COC and CC stretching vibrations were also identified at 1200–990/cm. In addition, in-plane ring deformation of CH and OH bending modes was observed in the spectrum (1400–1200/cm). Vibrations related to (1-3)-α-d-glucan were observed at ∼540/cm while the absorption corresponding to β-linked glycosidic bands was visible at 920–1060/cm. Table 3 summarizes the assignments of the different bands observed and discussed in this work. Figure 2 shows a semi-quantitative comparison of the main infrared bands present in both culture media, with respect to β-glucans and amide bands.
Functional groups resulting from curve fitting of the Kluyveromyces marxianus VM004 cell wall FITR spectra obtained using different culture media.
| Functional groups | Wavenumber/(cm) | |
|---|---|---|
| YPD | DDG | |
| OH stretching | 3400 | 3400 |
| CH stretching | 2940 | 2940 |
| OH2 stretching (CH2 of sugar) | 2853 | 2853 |
| Amide I (CO)+(NH) (indicative of chitine) | 1640 | 1640 |
| Amide II (NH)+(CN) (CO) (chitine) | 1562 | 1560 |
| CH2 in plane (CH2OH of sugar) | – | 1465 |
| In-plane ring deformation including CH and OH bending modes | – | 1400–1200 |
| (1-3)-α-d-Glucan | – | 1366 |
| COC and CC stretching vibrations (characteristic of polysaccharides) | 1200–990 | 1200–990 |
| Linear structure of β-d-glucan linked through (1-3) linkage | – | 1160 and 1074 |
| (1-3)(1-6)-β-d-Glucan | 1044 | 1060–1042 |
| COC and CC stretching vibrations (characteristic of polysaccharides) | 1200–990 | – |
| (1-3)(1-6)-β-d-Glucan | 1044 | – |
| (1-3)(1-6)-β-d-Glucan | 930 | – |
| (1-3)(1-6)-β-d-Glucan | – | 921 |
| β-Linked glycoside bands | 880 | 876 |
| Referred to anomeric structure around glycosidic bonds | – | 850 |
| (1-3)-α-d-Glucan | – | 850 |
| (1-4)(1-6)-β-d-Glucan | 765 | 770 |
| (1-3)-α-d-Glucan characteristic | – | 542 |
| (1-3)-α-d-Glucan characteristic | – | 454 |
| (1-3)-α-d-Glucan characteristic | – | 420 |
YPD: yeast extract-peptone-dextrose broth; DDG: dried distiller's grains with solubles.
The method was validated for linearity, accuracy, LOD and LOQ. Linearity of the method was tested by injecting three replicates (20μl) of three levels of AFB1 standard solutions (5–50ng/ml). The accuracy of the method was determined by a recovery assay as described above and the average content of AFB1 obtained was used to calculate the recovery percentage. The limit of detection (LOD) and limit of quantification (LOQ) for AFB1 were calculated based on signal-to-noise (S/N) ratios of 3:1 and 15:1, respectively, but were experimentally obtained injecting standard dilutions with the corresponding S/N ratio.
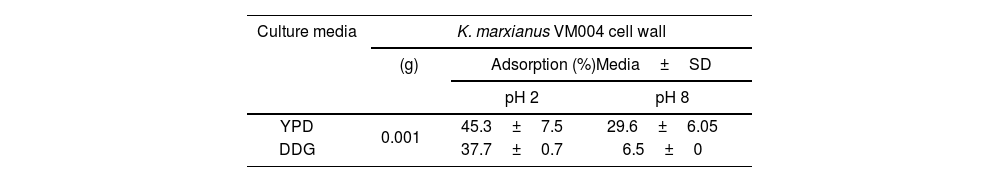
Table 4 shows the AFB1 adsorption percentage using the yeast cell and the CW. In general, the adsorption percentage was influenced by the assayed pH. At pH 2 the adsorption was higher than that at pH 8 in the two culture media studied.
Aflatoxin B1 adsorption by Kluyveromyces marxianus VM004 cell wall from different culture media and pHs.
| Culture media | K. marxianus VM004 cell wall | ||
|---|---|---|---|
| (g) | Adsorption (%)Media±SD | ||
| pH 2 | pH 8 | ||
| YPD | 0.001 | 45.3±7.5 | 29.6±6.05 |
| DDG | 37.7±0.7 | 6.5±0 | |
YPD: yeast extract-peptone-dextrose broth; DDG: dried distillers grains with solubles. The initial concentration was 159.3±10.6ng/ml.
The adsorption percentage (mean±SD) at pH 2 for K. marxianus VM004 grown in YPD broth was 8.40±1.11% and at pH 8 the percentage decreased until 1.63%. Cells grown in DDG adsorbed 13.8±2.3% at pH 2, while at pH 8 the adsorption was 5.2±1.8% (data not shown). The extracted CW obtained from YPD adsorbed 45.3±7.5% of AFB1 at pH 2, and 29.6±6.05% at pH 8. The extracted CW obtained from DDG adsorbed a lower percentage compared with YPD at both pHs studied. The CW adsorption from DDG at pH 2 was 37.7±0.7% and 6.5±0% at pH 8.
DiscussionThe present study evaluated the influence of two carbon sources on the ultrastructure and composition of the cell wall of K. marxianus VM004 using transmission electron microscopy and IR spectroscopy, respectively, and its effect on the adsorption of AFB1.
K. marxianus is a yeast widely used for different biotechnological applications, such as enzyme production, single cell protein, aromatic compounds, bioethanol, lactose reduction in foods, production of whey bioingredients, bioremediation, as anticholesterolemic agent, heterologous protein production, among others32. The K. marxianus strain used in this study for AFB1 adsorption has demonstrated probiotic properties, such as resistance to gastrointestinal conditions, aggregation, coaggregation to pathogenic microorganisms, production of antimicrobial substances and as a substitute growth promoter for antibiotics, improving health and productive parameters of weaned piglets4,14.
The intention of this study was to produce K. marxianus biomass in a reasonable way and compatible with commercial needs using an alternative medium such as DDG as a carbon source, which contained 2.67g/l of glucose, while the YPD broth contained 20g/l of glucose. Both culture media proved to be recommended for biomass and CW production. Yeast biomass was used for CW extraction in order to demonstrate AFB1 adsorption. The percentage of CW from K. marxianus in DDG was similar to that extracted by Nguyen et al.20 and higher than that extracted by Aguilar Uscanga et al.2. Nguyen et al.20 used a medium based on yeast extract (5g/l) and glucose (50g/l) and obtained values between 29.5 and 32.5% of CW and Aguilar Uscanga et al.2 used different carbon sources (glucose, mannose, galactose, sucrose, maltose and ethanol) and found that the percentage of CW in dry weight was 10% for the culture performed with 25% ethanol in the sucrose culture.
Mycotoxin contamination represents a global problem in the animal feed industry, and several methods for preventing mycotoxicosis are being studied. Yeasts have great potential to reduce the economic damage caused by mycotoxin intake. Several studies have been reported on the biodegradation and adsorption of mycotoxins using different yeast species mainly Saccharomyces cerevisiae8,15,21.
The use of K. marxianus as an adsorbent of nivalenol (NIV), zearalenone (ZEN) and deoxynivalenol (DON) has been demonstrated27. However, there are no reports of K. marxianus CW use as mycotoxin adsorbent. In this study, live cells and CW of K. marxianus VM004 were used to determine AFB1 adsorption.
In this study, the adsorption was influenced by pH. The adsorption at acid pH was higher than at alkaline one. The influence of pH was observed in previous studies using commercial yeast CW, demonstrating that at pH 2 the AFB1 adsorption was higher than at neutral pH23. Ye et al. showed that the adsorption of AFB1 by humic acid polymers is pH dependent and that the transition to alkaline conditions can lead to desorption35. Pereyra et al. studied the influence of pH on the conidia wall of Aspergillus niger aggregate and its relationship with ZEN adsorption24. They showed that at pH 2 the ZEA adsorption was lower than at pH 6. pH could act by changing the composition of the yeast wall or the chemical structure of the toxin. The effect of pH is difficult to explain; future research should focus on the study of the influence of pH on the chemical groups of the CW and mycotoxin.
In the present work, the adsorption of AFB1 using live cells and CW from K. marxianus VM004 in a simulated gastric and intestinal pH solution was studied. The percentage of AFB1 adsorption using only CW was higher than using the living cell. However, it is important to note that the use of the living cell would have a probiotic effect in addition to the AFB1 adsorbent. Yiannikouris et al. found that 6177μg/ml of AFB1 were adsorbed per 100μg/ml CW36. Pereyra et al. studied the adsorption of AFB1 with CW from commercial yeast (S. cerevisisae) applying mathematical models to explain the type of interaction of the toxin with the adsorbent23. They found values of 0.29 to 0.40 (g/g) at pH 2 and from 0.061 to 0.15 (g/g) at pH 6. These values are higher than those studied in our study at pH 2 and pH 8, but they are hypothetical values. Pereyra et al. studied the adsorption of AFB1 using CW of probiotic strains of S. cerevisiae and Saccharomyces boulardii, obtaining similar values to those in this study25.
Yeast CWs are composed mainly of polysaccharides, proteins and lipids that offer numerous functional groups for the interaction, such as carboxyl, hydroxyl, phosphate and amine groups, as well as hydrophobic adsorption sites, such as aliphatic chains and aromatic carbon rings22. For these reasons, the chemical structure of the toxin, the composition of the adsorbent and the pH of the medium where adsorption occurs must be taken into account to determine the adsorption efficiency.
It is known that the three-dimensional structure of the polysaccharides that make up the CW allows the adsorption of mycotoxins or their metabolic derivatives37,38. The interaction of mycotoxins with YCW is due to the helical conformation of (1-3)-β-glucans, which participate in Van der Waals bonds and hydrogen bonds, while (1-6)-β-glucans strengthen the Van der Waals bonds and stabilize the interaction36.
In this study, the TEM technique was used to evaluate the relationship between CW thickness and cell diameter, to determine the proportion of CW in K. marxianus strain VM004. This relationship shows an accurate estimate of the CW content. The cell diameter was almost double in DDG. The use of DDG increased the CW thickness of the strain; however, the ratio of CW thickness and cell diameter ratio was better using YPD.
In this study the CW composition of K. marxianus VM004 was evaluated using IR spectroscopy. This is a fast, precise and accurate method, requiring little sample preparation, for the determination and quantification of the carbohydrates that make up the YCW12,26. The functional groups present in CW can be identified by this technique because each group has a unique energy absorption band9. With this technique it was possible to study the carbohydrate variation of CW under the influence of two carbon sources (YPD and DDG broth). Furthermore, the IR spectroscopy analysis indicated the presence of CO, OH and NH groups, which are related to protein and carbohydrate components, mainly chitin and β-glucans involved in the adsorption of AFB1.
Taking into account the results of the IR spectroscopy, a semi-quantitative comparison of the main infrared bands present in both cell cultures was done. In this sense, the bands referred to glucan and its derivatives (region between 1400 and 800/cm of the infrared spectra) were present in a higher proportion for the yeast grown in the YPD broth. Moreover, the amide bands of chitin were observed at 1640 and 1560/cm. In this case, a similar behavior than that observed for glucan could be noted. Based on these results, it is possible to confirm that the presence of both biomolecules is higher in the case of yeast that is evaluated in YPD broth than in DDG broth.
In particular, we were focused on the identification of β-d-glucan and chitin of the K. marxianus VM004 strain. In our studies, it was determined that the proportion of β-glucan and chitin was higher in the cells grown in YPD than those in DDG. However, the percentage of CW and thickness of the CW was higher in the cells grown in DDG. Taking these results into account, the amount of CW and its composition were relevant for the adsorption of AFB1. Future studies should focus on the three-dimensional structure between β-glucan and chitin to determine how it affects AFB1 adsorption.
Additional studies are required to better describe the mechanism of adsorption. It is necessary to identify all the chemical structures and composition not only of the CW but also of the toxin and the environment where they interact. Yianninkouris et al. demonstrated that β-d-glucans are the yeast components responsible for the complexation of zearalenone (ZEN), and that the reticular organization of β-d-glucans and the distribution between β-(1-3)-d-glucans and β-(1-6)-d-glucans play a major role in the efficacy38. On the other hand, Jouany et al. defined the molecular indices of the binding ability of a single helix of β-d-glucans for AFB110. The aromatic ring, the lactone and ketone groups of AFB1 form polar or electron bonds with the glucose units in the single helix of β-d-glucans, which maintain the toxin linked to the glucans. In our study, the presence of the different types of glucans responsible for adsorption and the presence of chitin was determined. Although the study is semi-quantitative, a ratio of 1 was observed between chitin (as indicated by the amide bands) and β glucans in the CW extract obtained from YPD. While in the DDG, the CW extract showed a higher presence of chitin than of beta glucans. Chitin is responsible for the rigidity of the CW. However, a high amount of chitin hinders the adequate three-dimensional conformation of β glucans for the effective adsorption of the mycotoxin. In another work, the adsorption of ZEN was studied using four strains of S. cerevisiae with different ratios of total β glucans and chitin where adsorption was a dependent strain and taking into account the percentage of insoluble β glucans32.
In conclusion, this is the first time that the adsorption capacity of AFB1 by a CW obtained from a probiotic strain of K. marxianus VM004 is reported. In addition, the use of DDG as a carbon source could replace a synthetic medium such as YPD for the production of biomass and CW intended to be used as an adsorbent of AFB1. The use of K. marxianus VM004 CW as a mycotoxin adsorbent is a strategy to reduce animal exposure to mycotoxins.
Key contributionThere are no reports of K. marxianus cell wall use as aflatoxin B1 adsorbent. In this study, live cells and cell wall of K. marxianus were used to determine the AFB1 adsorption.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors are grateful to the Universidad Nacional de Rio Cuarto, Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-FONCYT) which supported this study through grants.