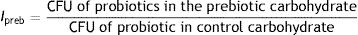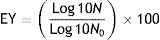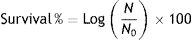The synergistic effect of microencapsulation in pectin microgels and inulin extracted from native crops of Jerusalem artichoke (JAI) was evaluated as a natural strategy to increase the survival of Lactobacillus paracasei subsp. tolerans F2 selected for its probiotic properties in Oncorhynchus mykiss. The strain was able to grow and ferment JAI in modified MRS broth, increasing cell population (∼+5 log units) with a net decrease in pH (6.2±0.2 to 4.0±0.5). Encapsulation of F2 in pectin microgels in the presence of JAI improved the survival of the strain not only during storage but also after exposure to simulated gastrointestinal conditions. Viable entrapped cells in the presence of the prebiotic were significantly higher (8.2–8.4log CFU/g) than without it (∼7.00log CFU/g) after 56 days at 4°C. These results encourage further implementation of these techniques for the formulation of functional feeds using natural alternative sources of inulin with greater viability on storage conditions and digestibility.
El efecto sinérgico de la microencapsulación en geles de pectina e inulina extraída de cultivos nativos de topinambur se evaluó como una estrategia natural para aumentar la supervivencia de Lactobacillus paracasei subsp. tolerans F2, bacteria seleccionada por sus propiedades probióticas sobre la trucha arco iris (Oncorhynchus mykiss). La cepa fue capaz de crecer y fermentar la inulina de topinambur en caldo MRS modificado, lo que condujo al incremento del número de células (∼+5 unidades logarítmicas) y la disminución neta del pH (6,2±0,2 a 4,0±0,5). La encapsulación de F2 en microgeles de pectina en presencia de inulina mejoró su supervivencia no solo a lo largo del almacenamiento sino también en condiciones donde se simuló el pasaje por el tracto gastrointestinal. Después de 56 días de almacenamiento a 4°C, se registraron números significativamente mayores de células viables microencapsuladas en presencia del prebiótico (8,2-8,4 log UFC/g) que sin él (∼7,00 log UFC/g). Estos resultados alientan la aplicación de estas técnicas para la formulación de alimentos funcionales utilizando fuentes alternativas naturales de inulina que incrementan la viabilidad del probiótico almacenado y su digestibilidad.
Prebiotics are non-digestible food ingredients that are selectively fermented by beneficial bacteria and allow specific changes, both in the composition and/or activity of the intestinal microbiota, conferring benefits to the host4. Because of their chemical structure these compounds are neither absorbed in the upper part of the gastrointestinal (GI) tract nor hydrolyzed by digestive enzymes. Among prebiotic polysaccharides, inulin-type fructans are well characterized for their physiological and biochemical features, maintaining healthy GI functions10. For the last 10 years, Jerusalem artichoke (Helianthus tuberosus L.) has been considered one of the most important candidates to be used as raw material for the industrial production of biological inulin due to its agronomic and industrial potential becoming an interesting crop at the regional level.
Furthermore, the combination of probiotics and prebiotics is known as symbiotic, which has additive or synergistic benefits with enhanced survival and implantation of live microbial cells in the host's gut3. Another strategy to enhance probiotic survival is microencapsulation which has been proposed as a useful technology to increase the survival of probiotic cells up to their ingestion, storage and through the GI tract6. Different types of polymers are proposed as coating materials, among them, pectin and its derivatives have proved to be potential prebiotics with improved properties causing positive effects in the distal part of the colon5.
The main objectives of the present work were (1) to screen the effect of inulin-rich carbohydrates (IRCs) extracted from native Jerusalem artichoke on Lactobacillus paracasei subsp. tolerans F2 growth and (2) to evaluate the combined effect of symbiotic and pectin microbeads to improve bacterial survival over GI conditions and refrigeration storage.
F2 isolated from the digestive tract of Ramnogaster arcuata and selected for its probiotic properties in Oncorhynchusmykiss7 was the chosen strain. The isolation methodology and molecular identification are described in Sica et al.12.
Food grade commercial inulin (CI) [(C6H10O6)n, carbohydrate content ≥90g/100g] was kindly donated by Granotec S.A. (Chile) and used as reference standard for comparison. Jerusalem artichoke inulin (JAI) was obtained from H. tuberosus L. tubers grown and harvested in Córdoba province (Argentina). The handling of Jerusalem artichoke tubers and inulin carbohydrate extractions were done as described in Rubel et al.11.
All experiments were carried out in triplicate, and the mean values are presented as means±standard deviations (SD). Data were analyzed using analysis of variance (ANOVA) followed by the Tukey's multiple comparison tests when necessary. Statistical significance was established at p˂0.05 for all the comparisons.
The prebiotic effect of inulin upon growth of F2 was analyzed in Man, Rogosa and Sharpe (MRS) modified broth supplemented with 1% (w/v) of CI (MRS-CI) and JAI (MRS-JAI) as principal carbon sources to supplement its growth. MRS supplemented with glucose (Merck, Germany) at 1% (w/v) (MRS-G) was employed as positive growth control, while MRS carbohydrate-free basal medium (MRS-B) was employed as negative control without any of the mentioned carbon sources. Inulin solutions were sterilized through 0.2μm filters (Gamafil S.A., Argentina) before being added to the corresponding growth media.
Assays in each modified MRS medium (MRS-CI, MRS-JAI, MRS-B and MRS-G) were performed with an initial cell density adjusted by optical density (OD) at 600nm of 0.250 (∼108cells/ml) using a spectrophotometer (Thermo Spectronic Genesys 20, Thermo Electron Corporation, USA). To screen the effect of inulin upon F2 growth, samples were withdrawn at a 2h interval along 24h for total viable counts in MRS agar. In all cases, the plates were incubated at 25±1°C for 48h and colony forming units (CFU)/ml were determined. The final pH of all cultures was measured. The incubation temperature never exceeded 25°C due to the sample origin and its potential use in aquaculture7,12.
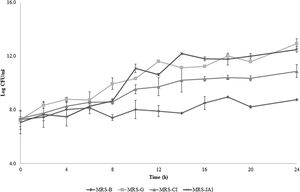
F2 had the ability to grow and ferment inulin with a significant pH decrease of 6.2±0.2 to 4.0±0.5 after 24h incubation. The fastest growth rate was achieved for MRS-G (1.22h−1) followed by MRS-JAI (1.33h−1), MRS-CI (5.71h−1) and MRS-B (7.43h−1). No significant differences (p>0.05) were evidenced when analyzing F2 viable counts after 24h incubation in the presence of glucose and JAI. As shown in Figure 1 log CFU of F2 obtained after 24h did not exhibit significant differences between MRS-G and MRS-JAI, being 12.94±0.37 and 12.50±0.21log CFU/ml, respectively, followed by MRS-CI with 10.89±0.49log CFU/ml. As expected MRS-B showed the lowest counts (8.77±0.03log CFU/ml, p˂0.01) as it lacked carbohydrates to support the growth.
Growth curves of Lactobacillus paracasei subsp. tolerans F2 in MRS supplemented with 1% (w/v) glucose (MRS-G), 1% commercial inulin (MRS-CI), 1% (w/v) Jerusalem artichoke inulin (MRS-JAI) and without carbohydrates as negative control (MRS-B). Growth curves were determined by viable counts expressed as log CFU/ml. Samples were incubated at 25±1°C for 24h.
The prebiotic index (Ipreb) was calculated as the ratio of probiotic growth in the chosen prebiotic to probiotic growth in a control carbohydrate2.
Ipreb expresses a quantitative value for comparison of different prebiotic carbohydrates8. The highest Ipreb was found for JAI with 0.97±0.01 followed by CI with 0.84±0.06. The Ipreb registered is almost 1 corresponding to the behaviour evidenced for glucose, being a good index for its replacement by an economical and natural resource.
Microcapsules were prepared by external cross-linking of a w/o emulsion1 with some modifications. Briefly, a 2% (w/v) pectin solution was prepared in distilled water at 50°C under magnetic stirring at 300rpm for 2h. The pectin used was low methoxyl Genu Pectin LM104 AS purchased from CPKelco (CPKelco, Limeira, Brazil) with a degree of esterification of 27% and degree of amidation of 20% of the original carboxyl groups. The solution was left to cool down until 30°C and the probiotic suspension prepared from F2 cultures suspended in sterile phosphate-buffered saline, PBS (0.15mol/l NaCl, 0.05mol/l KH2PO4, 0.05mol/l K2HPO4, pH 7.2) or PBS with 1% CI and JAI was added. The mixture was stirred for another 30min and then a w/o emulsion was prepared by blending 20ml of the former mixture with 20ml of commercial sunflower oil containing 1% (v/v) Tween 80 (Merck KGaA, Germany). A fine emulsion was generated using a homogenizer (Pro 250-Homogeneizer, ProScientific, USA) at a speed of 10000rpm for 3min. Microcapsules were then generated by adding 20g of calcium chloride solution (Anedra S.A., Argentina) (0.5%, w/w) in the form of w/o emulsion similar to that containing pectin and probiotics. This emulsion was added dropwise to the original w/o emulsion (40ml) under the lowest speed of the homogenizer and left stirring for another 30min.
The resulting microcapsules were recovered by a double washing-filtering sequence (Whatman® Grade 3 filter paper, provided by Sigma-Aldrich) by diluting 60g of the final emulsion with 250ml of distilled water containing 0.1% (v/v) Tween 80. Then, they were stored under refrigeration temperature at 4±1°C and protected from light.
Particle size distribution and the area basis mean diameter (D32) of the pectin microgels were obtained by analyzing the digital micrographs of the emulsions using the Image J 1.50i software [National Institute of Health (NIH), Bethesda, MD, USA]. The image was converted to grayscale (8-bit) and binarized (converted from grey level to black and white) using a thresholding method to differentiate the pectin microspheres. At least 3 micrographs were analyzed per sample so that more than 1000 particles were used.
Microgel particle sizes ranged roughly between 0.2 and 20μm with D32 of 7.48±1.30, 7.20±0.89 and 6.73±1.48μm for microcapsules with JAI, CI and without prebiotic, respectively.
Bacterial encapsulation efficiency was determined through the entrapment yield (EY) as stated in Reid et al.9:
where N is the number of viable entrapped cells released from the beads and N0 is the number of free cells added to the polymer mixture immediately before entrapment.To determine the entrapment of the different suspensions of F2, 0.1g of microcapsules were suspended in 0.1M sodium citrate buffer pH 6.2 for 20min followed by gentle shaking until complete dissolution at room temperature. Serial dilutions were performed and the bacterial contents of the capsules were determined by plating on MRS agar. EY of F2 in pectin microbeads was 92.3% without statistical differences between controls and particles containing IRCs.
To evaluate the protective potential of the prebiotic and microencapsulation over F2 survival through GI passage [simulated gastric (SGJ) and intestinal juice (SIJ)] assays were carried out in a two-step model.
Probiotic suspensions (∼108cells/ml) with 1% (w/v) JAI, CI or in complete absence of IRCs (controls) were inoculated in sterile SGJ composed of 125mM NaCl, 7mM KCl, 45mM NaHCO3 and 0.3% (w/v) pepsin (Saporiti S.A., Argentina), pH was adjusted to 2.0 with HCl (0.1mol/l). After 90min under low agitation, samples were centrifuged at 5000×g for 10min and washed twice with sterile PBS before being re-suspended in SIJ. SIJ was done by using sterile PBS containing pancreatin 0.09% (w/v) (Saporiti S.A., Argentina) and bile salt 0.6% (w/v), pH was adjusted to 8.0 using NaOH (0.1mol/l). After 90min, incubated cells were harvested by centrifugation and 100μl were re-suspended in 900μl of peptone water and serially diluted before plating in MRS agar for enumeration. In all cases the samples were incubated at 25±1°C for 48h.
The survival percentage of F2 after each GI step was calculated as:
JAI significantly improved F2 survival to simulated digestive solutions compared to CI and control (without carbon sources). After 1.5h in SGJ, F2+JAI exhibited 72.39% survival (6.11±0.03log CFU/ml) whereas F2+CI and F2-control the survival considerably dropped to 44.90 (3.79±0.69log CFU/ml) and 39.69% (3.35±0.72log CFU/ml), respectively. The same trend was evidenced after exposure to SIJ with a decrease of 0.20 log units for F2+JAI, 1.27 log units for F2-control and 1.56 log units for F2+CI. At the end of the passage through the GI tract, final viable cell counts were 5.91±0.26log CFU/ml for F2+JAI, 2.23±0.75log CFU/ml for F2+CI and 2.08±1.10log CFU/ml for F2-control.
For encapsulated cells, simulated GI challenge was performed at the end of the trial. For this purpose, a total of 1g of microparticles was analyzed following the same procedure as previously described for free probiotic cells. At the end of each step, the encapsulated cells were released from the particles for viable count, as previously described for EY.
The presence of IRCs increased the resistance of F2 to SGJ exposure; viable cell reductions were 16% and 18% in the presence of JAI and CI, respectively, versus a 23% cell drop for F2 without prebiotics (F2-control). Similarly, at the end of the SIJ step, the encapsulated F2 cells without prebiotics showed the lowest survival, 4.66±0.01log CFU/g, whereas the presence of IRCs significantly increased their resistance with viable counts of 6.76±0.33log CFU/g for F2+JAI followed by F2+CI with 5.74±0.12CFU/g. No significant differences between the IRCs used were observed.
Furthermore, microencapsulation with pectin and JAI as prebiotic is an excellent tool to preserve the concentration and viability of probiotic cells until they reach the intestine.
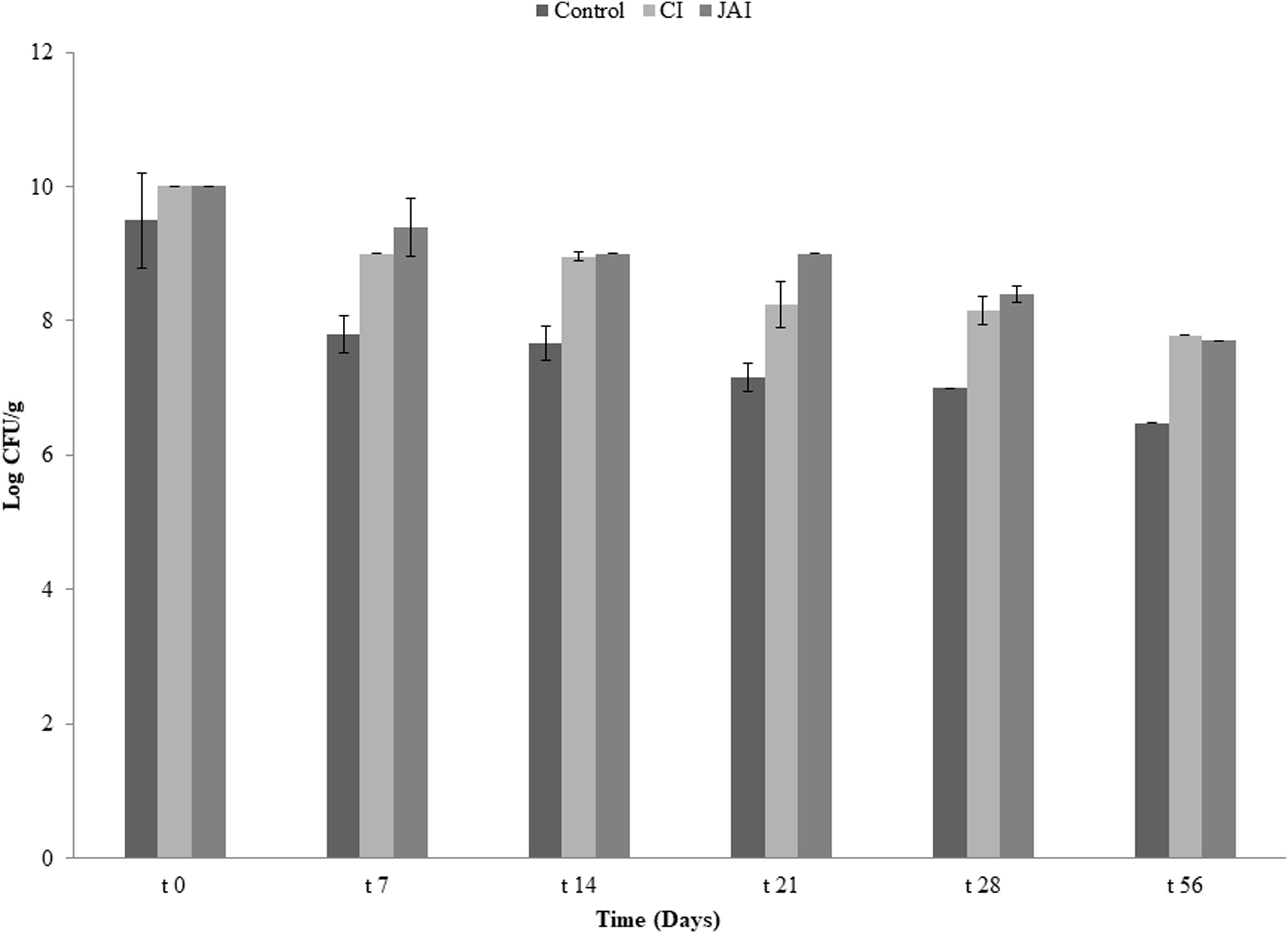
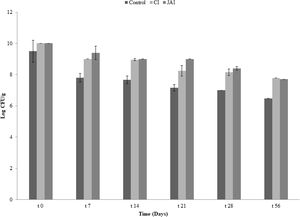
To evaluate the combined effect of symbiotic and pectin microbeads to improve bacterial survival in refrigeration storage, pectin microparticles were kept at refrigeration temperature (4°C) for an eight-week (∼56 days) interval. The number of culturable probiotic cells was determined once a week. Supplementary Figure 1 shows that the survival rate of encapsulated F2 cells in the presence of IRCs over time was significantly higher (p˂0.01) than for free probiotic cells. The viability of F2 entrapped in beads continuously decreased from day 0 to day 56: 2.50, 1.85 and 1.61 log units for control, CI and JAI, respectively.
It was observed that microparticles with the addition of JAI as prebiotic, prepared by emulsification/internal gelation using pectin, represent an efficient system for promoting the resistance of F2 during the simulation of passage through the GI tract, also being an efficient means to promote viability of the microorganism at 4°C for 56 days.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, Fondo para la Investigación Científica y Tecnológica (FONCYT), Ministerio de Ciencia, Tecnología e Innovación de la Argentina (PICT 2015, No. 0156) for funding this research and Dr. Rubel Irene for kindly providing the inulin used from Jerusalem artichoke tubers.