The application of pyrethroids and carbamates represents an environmental risk and may exert adverse effects on beneficial microorganisms such as Trichoderma, which contribute to the biocontrol of several fungal phytopathogens. This research evaluated the tolerance of several strains of Trichoderma to a selected culture medium contaminated with a commercial insecticide (H24®) composed of pyrethroids, permethrin and prallethrin, and carbamate propoxur, and determined the influence of this insecticide on the release of enzymes such as chitinases, peroxidases, and endoglucanases by a consortium of selected Trichoderma strains grown in liquid culture medium. Four out of 10 Trichoderma strains showed tolerance to 200ppm (∼48.3% of growth) of the commercial insecticide after 96h of exposure to a contaminated solid medium. After eight days of growth in liquid culture, the insecticide enhanced extracellular protein content and peroxidase activities in the Trichoderma consortium but decreased both chitinase and glucanase activities. These fungal responses should be considered when implementing strategies that combine alternative pesticides and fungal biocontrollers for managing fungal phytopathogens.
La aplicación de piretroides y carbamatos representa un riesgo ambiental y puede ejercer efectos adversos sobre microorganismos benéficos, como el Trichoderma, que contribuyen al biocontrol de varios fitopatógenos. Por un lado, esta investigación evaluó la tolerancia de varias cepas de Trichoderma a un medio de cultivo sólido contaminado con un insecticida comercial (H24®) compuesto por piretroides (permetrina y praletrina) y carbamato propoxur; por el otro, determinó la influencia de este insecticida en la liberación de enzimas como quitinasas, peroxidasas y endoglucanasas por un consorcio de cepas seleccionadas de Trichoderma cultivadas en medio líquido. Cuatro de 10 cepas de Trichoderma mostraron tolerancia a 200 ppm (∼48,3% de crecimiento) del insecticida comercial después de 96 horas en un medio sólido contaminado. Tras ocho días de crecimiento en cultivo líquido, el insecticida aumentó el contenido de proteínas y la actividad peroxidasa del consorcio Trichoderma, pero redujo las actividades quitinasa y glucanasa. Estas respuestas fúngicas podrían ser consideradas al implementar estrategias para el biocontrol y el manejo de hongos fitopatógenos.
After the prohibition of using the highly toxic and persistent dichloro diphenyl trichloroethane (DDT), alternate insecticides based on carbamates and pyrethroids (single or combined) began to be utilized for controlling insect pests17. However, these insecticides may also represent potential issues for human and environmental health47. Some carbamates such as propoxur or pyrethroids such as permethrin and prallethrin may have carcinogenic and mutagenic properties, and inflammatory effects in the stomach; in addition, they are also associated with alterations in functional sodium channels and poor motor development in children25,30,44. Furthermore, several studies reported the effect of carbamates and pyrethroids on beneficial bacteria and communities in the rhizosphere10,35,36,38,47. Therefore, research focused on improving strategies for integrated pest management, which includes the combination of organically-derived pesticides and the application of antagonist microorganisms to reduce chemical damage induced by insecticides in the environment38.
Trichoderma genus includes worldwide ubiquitous fungi commonly found in soils and possesses a powerful and versatile enzymatic machinery (including cellulases, chitinases, peroxidases, and proteases, among others) which may degrade a wide range of organic recalcitrant compounds in soil3,29,41. Commonly, Trichoderma is one of the widest fungi directed to control plant pathogens since they produce enzymes with hydrolytic capacity and secondary metabolites related to processes such as antibiosis, space competition, plant growth improvement, and resistance against biotic and abiotic stresses27,32,37. Furthermore, Trichoderma spp. are tolerant to many agrochemicals and have the potential to degrade chemical pesticides because they possess specific enzymes to metabolize such compounds8.
Therefore, the aims of this research were (1) to determine the tolerance of several strains of Trichoderma to solid culture medium contaminated with commercial insecticide H24® (composed of pyrethroids, permethrin and prallethrin, and carbamate propoxur) and (2) to evaluate the effect of this insecticide on the release of enzymes such as chitinases, peroxidases, and endoglucanases by selected Trichoderma strains grown in liquid culture medium.
Materials and methodsMicrobiological materialsThis research utilized ten strains of Trichoderma: Trich CP01 (T. virens), Trich CP03 (T. koningii), Trich CP04 (T. viride), Trich CP022 (T. virens), Trich CP023 (T. koningii), Trich CP037 (T. virens) Trich CP038 (T. harzianum), Trich CP056 (T. viride), Trich CP0X (T. atroviride), Trich CP0TGC (T. viride), and one strain of Phanerochaete chrysosporium-ATCC 34540 (CDBB 686) as referential fungus able to degrade toxic organic contaminants.
All Trichoderma strains are part of the microbial collection of the Microbiology Laboratory (Colegio de Postgraduados), which were reported as tolerant to crude oil, and to high concentrations of naphthalene, phenanthrene, and benzo[a]pyrene2. The strain of P. chrysosporium-ATCC 34540 (CDBB 686) was acquired from the CINVESTAV microbial repository and reported as tolerant and degrader of persistent organic pollutants, including insecticides24.
Chemical reagents and culture mediaThe commercial insecticide H24® contains permethrin (360mg/kg), propoxur (890mg/kg) and prallethrin (50mg/kg) as active ingredients; thus, the total of active ingredients yields up to 1300mg/kg, which are dissolved in an organic solvent. This commercial insecticide is commonly applied for controlling flying and crawling insects that attack cotton crops due to its active ingredient permethrin. Potato dextrose agar (PDA) medium (BD Bioxon®) was prepared according to the manufacturer's specifications and different concentrations of the commercial insecticide (0, 50, 100, 150, and 200ppm) were added to it.
The liquid culture consisted of a mineral medium (MM) prepared in accordance with Gao et al. with some modifications13. The MM contained (g/l): 1.0 K2HPO4, 0.5 KCl, 0.5 MgSO4·7H2O, 0.01 FeSO4, pH 7.0. The nitrogen source was meat peptone (CAS 91079-38-8, Merck), considering a nitrogen content of ∼12.5% and nitrogen in the insecticide (propoxur), the carbon source was sucrose and that carbon derived from the insecticide. The final C/N ratio was ∼20/1, considering 100ppm of active ingredients (permethrin, prallethrin, and propoxur) in commercial insecticide H24®. Both media were autoclaved at 121°C for 15min; afterwards, the H24® insecticide was added using filtration.
Bioassay 1. Fungal growth and tolerance to increased concentrations of commercial insecticideAgar disks of 5mm in diameter with fungal mycelium were sown in Petri dishes containing solid PDA medium with or without the commercial insecticide (four replicates per treatment); all fungal cultures were incubated at 28°C for 96h. The growth diameter was measured every 24h, for 4 days. The results were used to estimate the radial growth, radial growth rate, radial growth rate inhibition (%) (RGRI%), and the inhibitory dose 50 (ID50)28,34. The radial growth rate was calculated with the quadratic equation that was fitted to the dose–response curve for each strain. The radial growth rate was used to estimate the (ID50) for each strain and the RGRI%28,34. The ID50 was also utilized as a reference to define a sub-ID50 without inhibiting the growth and further estimations33.
Bioassay 2. Fungal protein content and induced peroxidase, chitinase, and glucanase activities in a liquid medium containing/contaminated with 100ppm of commercial insecticideFour tolerant strains of Trichoderma sp. (a consortium of Trichoderma sp.) selected from Bioassay 1, and P. chrysosporium-ATCC 34540 were used for evaluating the effect of a sub-ID50 of commercial insecticide H24® on the enzymatic activities of interest.
The initial fungal inoculum was adjusted for applying 1×106 spores ml of the Trichoderma consortium (adding the same number of spores per each fungal strain), and 1×106 spores ml of P. chrysosporium-ATCC 34540. Fungal cultures were maintained for 8 days at 200rpm and 28°C. Afterwards, protein analysis and enzyme tests were performed.
Protein content was determined with the Biuret method by using bovine albumin as standard14. The reaction mixture contained 100μl of fungal supernatant and 1000μl of Biuret reagent. This mixture was incubated for 30min at room temperature (20–25°C). Then, absorbance readings at 540nm were taken by using a spectrophotometer (Synergy 2, Biotek®).
Non-specific peroxidase (POX, EC 1.11.1.7) activity was measured in 96-well microplates by mixing 20μl of the fungal supernatant with 190μl of phosphate buffer (50mM, pH 7.0), 10μl of guaiacol 1%, and 20μl of H2O2 in phosphate buffer (50mM, pH 7.0). Absorbance readings at 450nm were taken from this mixture every 15s for 5min, by using a spectrophotometer (Synergy 2, Biotek®). POX activity was calculated using a molar extinction coefficient (ɛ) of 16.8/mMcm. One unit of POX activity is defined as the amount of enzyme that catalyzes the formation of 1μmol of tetraguaiacol per min at 25°C and pH 7.07.
Chitinase activity (GlcNAc activity) was measured by using the method proposed by Vargas-Hoyos and Gilchrist-Ramelli, using N-acetyl-d-glucosamine (GlcNAc) as standard22,42. Thus, 500μl of fungal supernatant was mixed with 100μl of colloidal chitin in sodium citrate buffer (50mM, pH 5.2), and incubated for 30min at 40°C; then, the mixture was cooled at ambient temperature (20–25°C), and 2000μl of dinitrosalicylic acid (DNS) were added, for further incubation at 90°C for 5min. The reaction mixture was cooled with iced water, and absorbance readings were measured at 540nm in a Synergy 2, Biotek® spectrophotometer. One unit of GlcNAc activity was defined as the amount of the enzyme necessary to liberate 1μmol/min of reducing sugar (glucose). Enzyme activity was expressed in units per mg of protein.
β-1,4-Glucanase activity (CMCase activity) was quantified by the DNS technique using d-glucose as standard15,22. The reaction mixture consisted of 500μl of carboxymethyl cellulose (2%) in sodium citrate buffer (50mM, pH 4.8) and 500μl of fungal supernatant. The mixture was incubated for 30min at 50°C and cooled at room temperature. Then, 5000μl of DNS solution was added and incubated at 90°C for 5min; the reaction was stopped by adding iced water. Absorbance readings were taken at 540nm in a spectrophotometer (Synergy 2, Biotek®). One unit of CMCase activity was defined as the amount of the enzyme necessary to liberate 1μmol/min of glucose, and the enzyme activity was expressed in terms of units per mg of protein.
Statistical analysisData were analyzed by one-way ANOVA and by the mean comparison test (Tukey, α=0.05). Data represents the values of the means±standard error (SE), from four replicates for each treatment. Analyses were performed using SPSS software, version PASW 18 (IBM SPSS-IBM Corp).
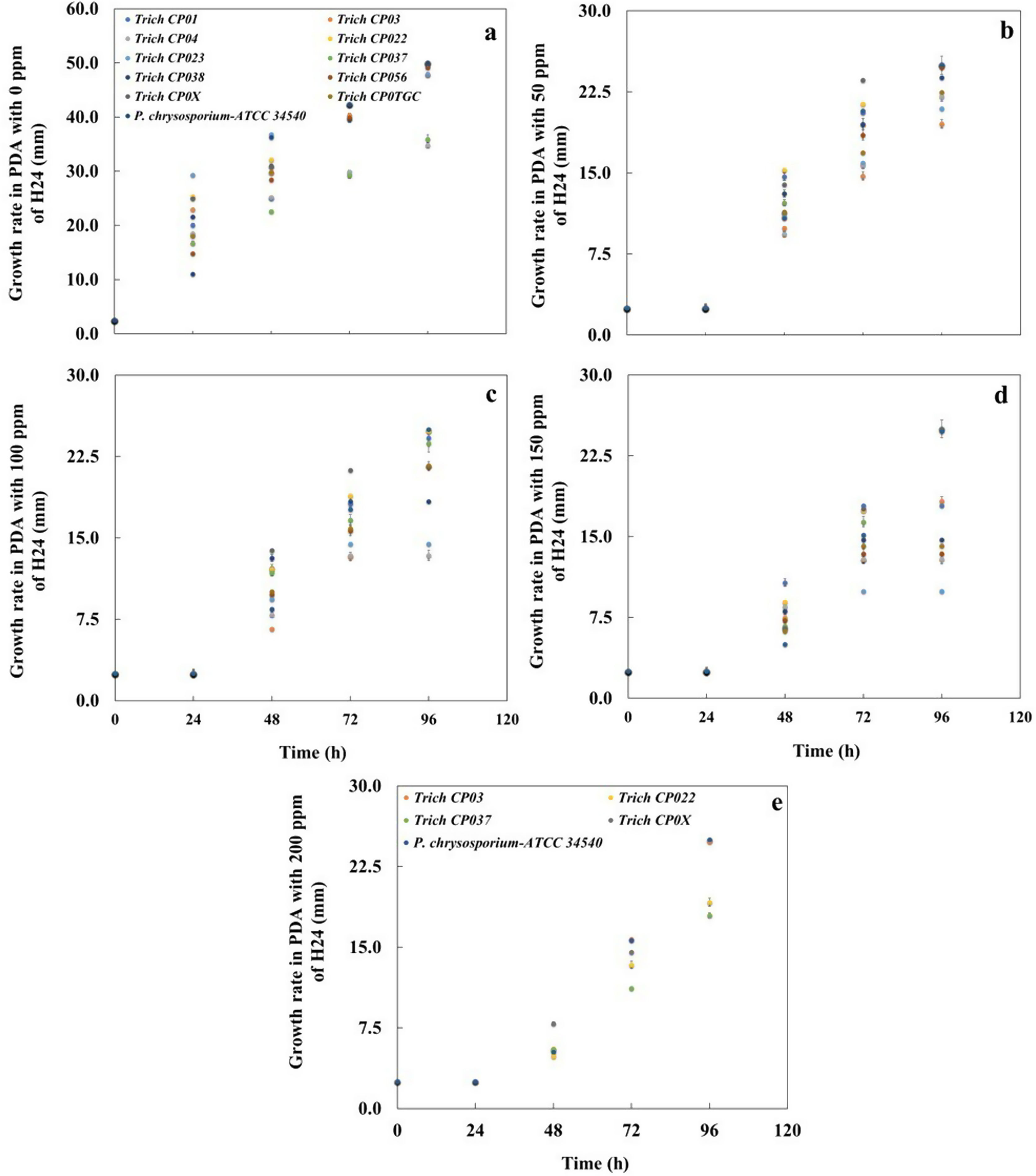
ResultsBioassay 1. Fungal growth and tolerance to three concentrations of commercial insecticideIn general, all Trichoderma strains tolerated the presence of the commercial insecticide H24® when applied at 50, 100, and 150ppm. Nevertheless, the strains CP01 (Trichoderma virens), CP04 (T. viride), CP038 (T. harzianum), CP056 (T. viride), and CP0TGC (T. viride) stopped their growth after 72h of exposure to 150ppm of the commercial insecticide (Figs. 1b–d). Only four Trichoderma strains (CP03 T. koningii, CP022 T. virens, CP037 T. virens, and CP0X T. atroviride) tolerated the application of the insecticide at 200ppm (Fig. 1e). Fungal adaptation to the insecticide occurred after 24h when all Trichoderma strains exposed to 50, 100, 150, and 200ppm showed visible mycelial growth (Figs. 1b–e).
Growth rate of Trichoderma strains and Phanerochaete chrysosporium-ATCC 34540 in solid medium (PDA) with (a) 0mg/l, (b) 50mg/l, (c) 100mg/l, (d) 150mg/l, and (e) 200mg/l of commercial insecticide H24® with three active ingredients (permethrin, prallethrin, and propoxur). Means±standard error (n=4).
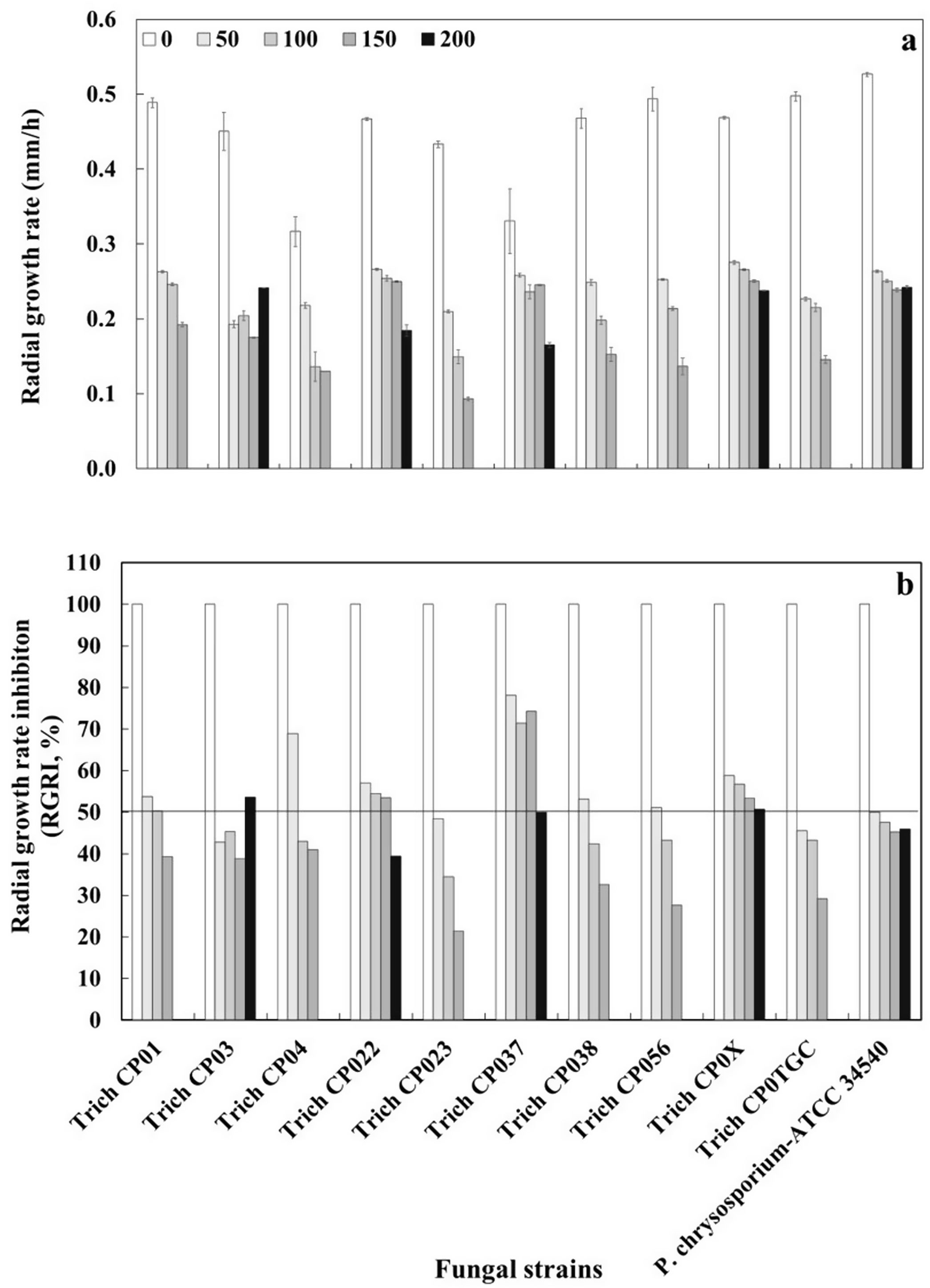
The growth rate of Trichoderma strains and P. chrysosporium-ATCC 34540 in a solid medium (PDA) containing 0, 50, 100, 150, and 200ppm of active ingredients (permethrin, prallethrin, and propoxur) of the commercial insecticide was determined. The tested concentrations had a negative effect on the radial growth rate of all Trichoderma strains and P. chrysosporium-ATCC 34540 when compared to their respective control without insecticide. The radial growth rate achieved at 150ppm oscillated between ∼0.50 and ∼0.09mm/h, while the radial growth rate for tolerant strains at 200ppm was between ∼0.53 and ∼0.17mm/h (Fig. 2a). Trich CP037 (T. virens) showed the lowest radial growth rate when exposed to 200ppm of the commercial insecticide (Fig. 2).
(a) Radial growth rate (mm/h) and (b) radial growth rate inhibition (RGRI%) of Trichoderma strains and Phanerochaete chrysosporium-ATCC34540 in solid medium (PDA) with 0, 50, 100, 150, and 200mg/l of commercial insecticide H24® with three active ingredients (permethrin, prallethrin, and propoxur). Means±standard error (n=4).
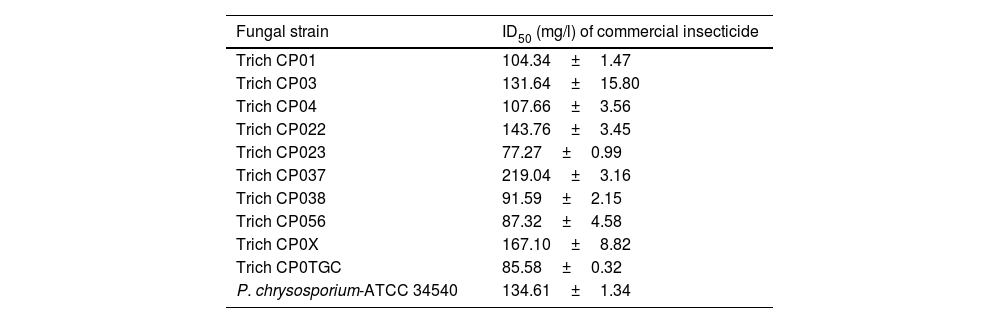
Data from radial growth rate and radial growth rate inhibition (%) were used to obtain the inhibitory dose 50 (ID50) of the commercial insecticide (Table 1). ID50 showed variations among all fungal strains. The values of the ID50 for those tolerant strains (Trich CP01; Trich CP04; Trich CP023; Trich CP038; Trich CP056; Trich CP0TGC) exposed to 150ppm ranged from 77.27 to 107.66ppm. Moreover, the values of the ID50 for those tolerant strains (Trich CP03; Trich CP022; Trich CP037; Trich CP0X; and P. chrysosporium-ATCC 34540) exposed to 200ppm ranged between 131.64 and 219.04ppm; of these strains, Trich CP037 showed the highest ID50 value (219.04ppm) when compared to that from P. chrysosporium-ATCC 34540 (Table 1).
Inhibitory dose 50 (ID50) of commercial insecticide H24® with three active ingredients (permethrin, prallethrin, and propoxur) on the growth rate of ten strains of Trichoderma sp. and Phanerochaete chrysosporium-ATCC 34540 grown on solid culture medium.
| Fungal strain | ID50 (mg/l) of commercial insecticide |
|---|---|
| Trich CP01 | 104.34±1.47 |
| Trich CP03 | 131.64±15.80 |
| Trich CP04 | 107.66±3.56 |
| Trich CP022 | 143.76±3.45 |
| Trich CP023 | 77.27±0.99 |
| Trich CP037 | 219.04±3.16 |
| Trich CP038 | 91.59±2.15 |
| Trich CP056 | 87.32±4.58 |
| Trich CP0X | 167.10±8.82 |
| Trich CP0TGC | 85.58±0.32 |
| P. chrysosporium-ATCC 34540 | 134.61±1.34 |
Active ingredients in the commercial product (H24®) are permethrin, prallethrin, and propoxur. Means±standard error (n=4).
In addition, ID50 was used for a proposed sub-ID50 for those fungal strains that tolerated 200ppm of the commercial insecticide. This sub-ID50 was utilized for Bioassay 2 to determine the enzyme activities from a Trichoderma sp. consortium and P. chrysosporium-ATCC 34540.
Bioassay 2. Fungal growth, protein content, and induced peroxidase, chitinase, and glucanase activities in a liquid medium containing 100mg/l of commercial insecticideThe Trichoderma consortium [Trich CP03 (T. koningii), Trich CP022 (T. virens), Trich CP037 (T. virens), and Trich CP0X (T. atroviride)], and P. chrysosporium-ATCC 34540, were exposed to 100ppm of the active ingredient of the commercial insecticide. After eight days, either the Trichoderma sp. consortium or P. chrysosporium grew and released proteins and enzymes into the contaminated liquid culture (Figs. 3 and 4).
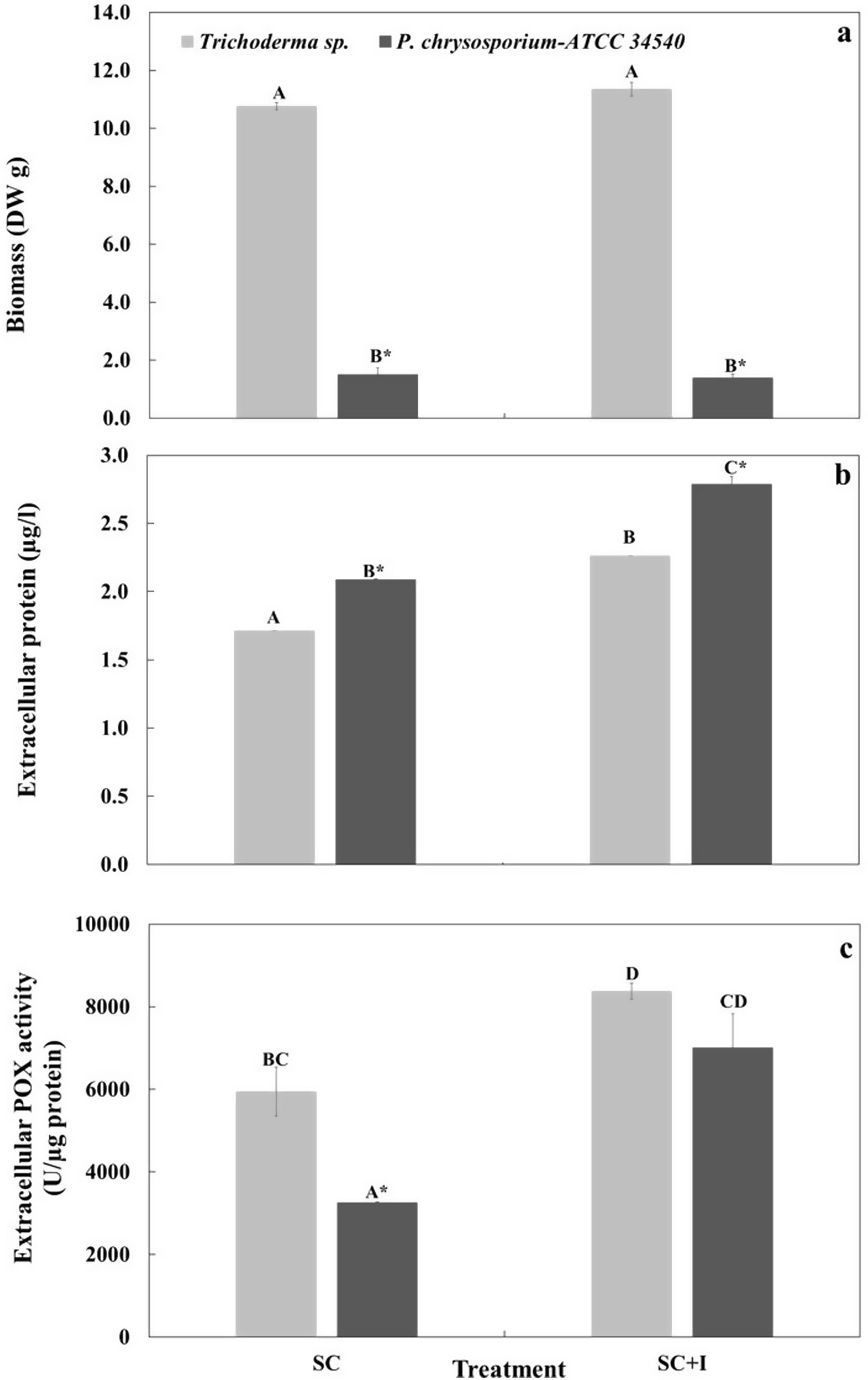
(a) Biomass (DWg), (b) extracellular protein content (μg/l), and (c) extracellular peroxidase activity (POX activity, U/μg protein) of Trichoderma sp. and P. chrysosporium-ATCC 34540 in liquid culture in the absence (SC) and presence (SC+I) of sub-ID50 (100mg/l) of commercial insecticide H24® with three active ingredients (permethrin, prallethrin and propoxur), after 8 days in liquid culture. Different letters indicate significant differences among means for medium culture; asterisks indicate significant differences among means for microorganisms (Tukey, α=0.05). Means±standard error (n=4).
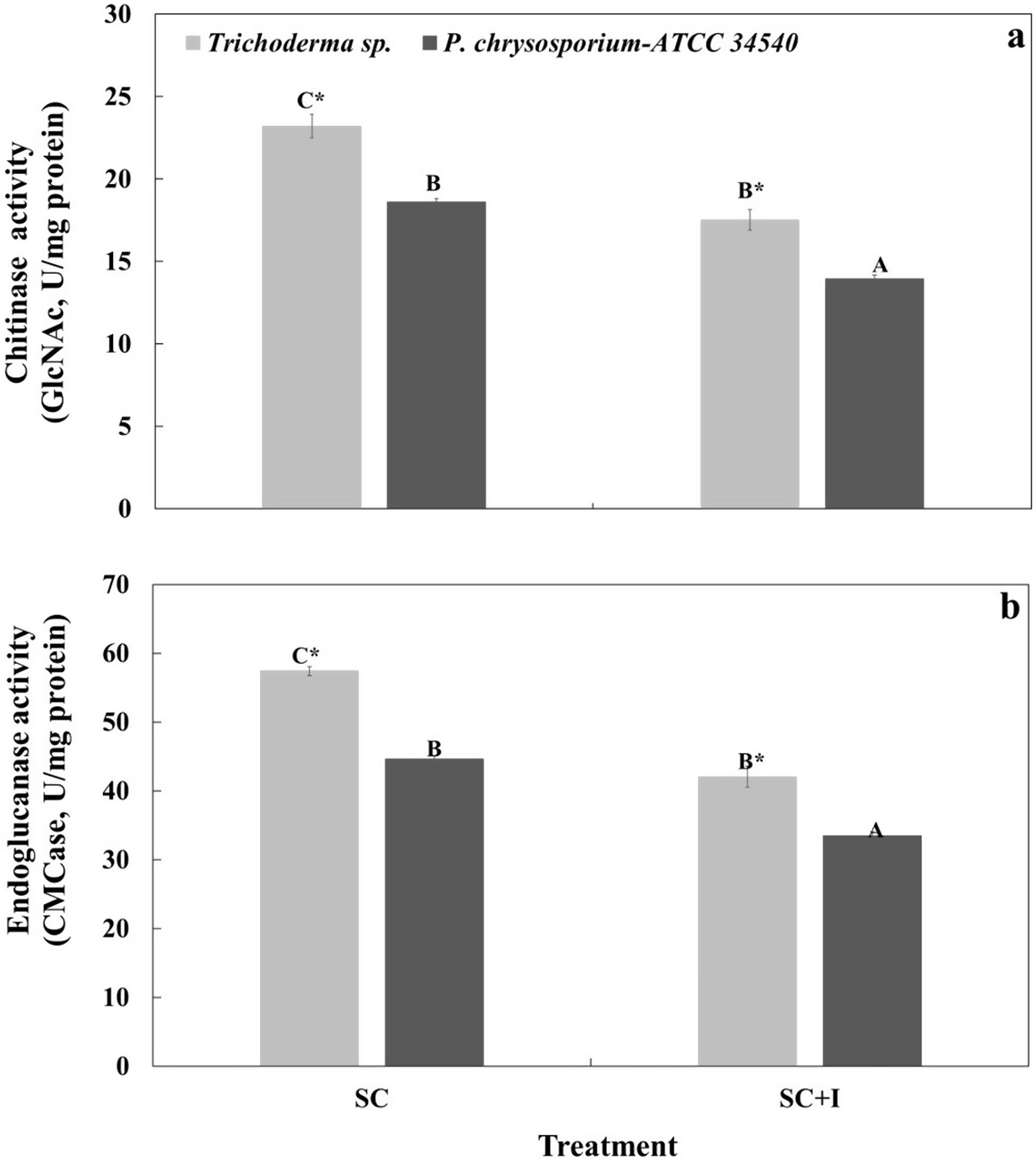
(a) Chitinase activity (GlcNAc, U/mg protein) and (b) endoglucanase activity (CMCase, U/μg protein) of Trichoderma sp. and P. chrysosporium-ATCC 34540 in liquid culture in the absence (SC) and presence (SC+I) of sub-ID50 (100mg/l) of commercial insecticide H24® with three active ingredients (permethrin, prallethrin, and propoxur), after 8 days in liquid culture. Different letters indicate significant differences among means for medium culture; the asterisk indicates significant differences among means for microorganisms (Tukey, α=0.05). Means±standard error (n=4).
In the present study, we observed that the growth of the Trichoderma consortium and P. chrysosporium was not affected by the active ingredients contained in the commercial insecticide (Fig. 3a). However, in the absence and presence of insecticides, the biomass of the Trichoderma sp. consortium was significantly higher (∼7-fold) than that of P. chrysosporium.
The strain of P. chrysosporium-ATCC 34540 produced more protein than the Trichoderma consortium in the absence or presence of the insecticide. The insecticide significantly influenced the protein production in the Trichoderma consortium and P. chrysosporium, whose protein content increased by ∼1.3-fold under the contaminated culture (Fig. 3b).
On the other hand, without the insecticide, the Trichoderma consortium had significantly higher (∼1.8-fold) POX activity than P. chrysosporium, reaching levels of ∼6000U/μg protein when compared to P. chrysosporium (∼3250U/μg protein) (Fig. 3c). Under insecticide contamination, POX activity increased in both fungal cultures; for the Trichoderma consortium, an increase of POX was achieved (∼1.4-fold), whereas for P. chrysosporium the increase of POX was about ∼2.1-fold than that of the respective control in the absence of the insecticide. Overall, the POX activity of the Trichoderma consortium was slightly higher (∼1.2-fold) but not significant, as determined for P. chrysosporium (Fig. 3c).
The chitinase activity (GlcNAc activity) for the Trichoderma consortium was higher (∼1.2-fold) than that achieved for P. chrysosporium, either in the absence or presence of the insecticide (Fig. 4a). Overall, the application of the insecticide caused a 25% decrease in the GlcNAc activity of both fungal cultures; however, the GlcNAc activity in the Trichoderma consortium was always significantly higher (∼1.2-fold) than that in P. chrysosporium (Fig. 4a).
In the absence of the insecticide, the CMCase activity for the Trichoderma consortium was significantly higher (∼1.4-fold) than the CMCase activity achieved by P. chrysosporium. The insecticide significantly decreased the CMCase activity of both fungal cultures; overall, the enzyme activity of the Trichoderma consortium was significantly higher (∼1.3-fold) than that of P. chrysosporium (Fig. 4b).
DiscussionMany microorganisms grow in the presence of pesticides, and this ability is influenced by chemical, physical, biochemical, and environmental conditions, and also depends on the amount and type of pesticides36. The initial fungal response to contaminants reflects the initial adaptation to stressful cultural conditions and/or to contaminated environments20. Synthetic pyrethroids (cypermethrin, deltamethrin, permethrin, and others) and carbamates such as propoxur may reduce the growth of bacteria and filamentous fungi such as T. viride, T. harzianum, and P. chrysosporium strains1,10,23,36,47. Conversely to our results, Schumacher and Poheling did not find negative effects of permethrin on the growth of Metarhiziumanisopliae35. On the other hand, pyrethroids such as allethrin (50mg/l) did not affect the growth of Fusarium proliferatum CF24. Consistently to our results, Deng et al. observed that pyrethroids such as β-cypermethrin (100mg/l) did not affect the final biomass produced by Aspergillus niger YAT; however, its radial growth was delayed10.
The mixture of pyrethroids, β-cypermethrin, deltamethrin, fenvalerate, and α-cyhalothrin (100–1000mg/l), reduced the growth in T. viride and P.chrysosporium36. In soils, the application of cypermethrin and chlorpyrifos alone and in combination dramatically decreased both bacterial and fungal populations39. The latter indicates that microorganisms require a certain period of adaptation to toxic contaminants to produce those necessary molecules for tolerating such compounds1. In the present study, the commercial product has a mixture of two pyrethroids (permethrin, prallethrin) with carbamate (propoxur), and this combination exerts certain toxicity which significantly delayed the growth of all fungal strains. This effect could be due to the synergy between carbamates and pyrethroids by which the toxicity to microbes may increase17.
Some studies have demonstrated the ability of Trichoderma strains for tolerating and growing in the presence of organic compounds such as petroleum hydrocarbons and pyrethroids2,6,36. Furthermore, these fungi may use organic molecules as carbon and energy source since they have the enzymes necessary to perform such metabolism18,45. The fungus identified as part of the genus Cladosporium was reported as tolerant to pyrethroids, including β-cypermethrin, deltamethrin, bifenthrin and permethrin (100mg/l)8. Other studies include fungi such as Aspergillus oryzae and Cunninghamella elegans, exposed to pyrethroids, cyhalothrin and 3-phenoxybenzoic acid, an intermediate in the degradation of permethrin31,49.
Consortium cultures are better than individual cultures because there are complementary physiological and biochemical functions among microorganisms, i.e., while some microorganisms perform specific enzymatic activity, other microorganisms can perform some different enzyme activities, by which all the involved organisms may be benefited40. Enzyme activities include superoxide dismutases (SOD), peroxidases (POXs), catalases (CAT), chitinases, glucanases, and many others.
In nature, microorganisms coexist in consortia and interact with each other to transform organic materials26,40. Artificial and natural microbial consortia are being studied for assessing tolerance, removal, and degradation of inorganic and organic compounds40. However, little attention is given to filamentous fungi and their tolerance to insecticides as accounted for bacteria9. Moreover, research about fungal consortia exposed to pyrethroids and carbamates utilized as substitutes for DDT is scant. Furthermore, many fungi may remove or degrade inorganic and organic compounds from polluted systems through several biochemical processes which include antioxidant molecules and enzymes such as POXs. In this regard, POXs have antioxidant activity and are involved in the detoxification of reactive oxygen species (ROS) such as H2O2 accumulation in cells because these enzymes oxidize H2O2 and are involved in the detoxification processes of organic pollutants19,46. Other extracellular fungal POXs (lignin peroxidase, manganese peroxidase, and versatile peroxidase VP) are involved in the removal and degradation of pollutants such as polycyclic aromatic hydrocarbons (PAHs), dye-based textile effluents, polychlorinated biphenyls, fungicides, and pesticides46.
Our results concur with those findings in white-rot fungi such as Polyporus tricholoma, Cilindrobasidium leave, and Deconica citrispora that increased POX activity (especially MnP) when exposed to paraquat, a widely applied herbicide in agriculture whose chemical structure resembles that of lignin5. In addition, P. chrysosporium can degrade a wide variety of organic pollutants due to the activity of non-specific extracellular POXs24. Furthermore, non-ligninolytic fungi such as Aspergillus, Fusarium, and Trichoderma may transform environmental pollutants such as PAHs, pesticides, and dyes, releasing POX enzymes21. Zhao et al. proposed that A. oryzae M4 uses some POXs in the presence of NADPH and O2, for degrading 3-phenoxybenzoic acid, a subproduct of permethrin degradation48. Trichoderma asperellum H15 was exposed to PAHs with 3–5 rings (phenanthrene, pyrene, and benzo[a]pyrene), and the fungal POX activity increased after 4 days of exposure46. Many POXs were identified in T. asperellum, including cytochrome C peroxidases, catalases, glutathione peroxidase, and dye-decolorizing peroxidases46. Most fungi, including P. chrysosporium and Trichoderma sp., are studied for degrading or removing PAHs, organochloride pesticides, organophosphates, carbamates, and pyrethroids such as β-cypermethrin and deltamethrin, by means of the oxidative effects of POXs11,12,24,43. In our results, the POX activity detected in the Trichoderma consortium was higher than that of P. chrysosporium, which may be explained in part, by the fact that the Trichoderma consortium was integrated by four fungal strains, and thus, all strains may have released more POX enzymes.
The studies of POXs involved in either the removal or degradation of pyrethroids such as permethrin and prallethrin, and carbamates such as propoxur, are still little explored. Our results suggest that the Trichoderma consortium and P. chrysosporium increase their POX activities for tolerating the commercial insecticide in liquid culture. However, further studies are needed to identify specific POXs that participate during this process, and to determine whether these enzymes act on both the degradation and detoxification of insecticides and byproducts, as well.
On the other hand, chitinases play a key role in the transformation of chitin and are widely distributed in nature with a wider range of biotechnological applications, including the biocontrol of fungal phytopathogens, harmful insects, bioconversion of chitin wastes, to single-cell protein, biopesticides, among others37. Moreover, chitinases are important for maintaining the balance of carbon and nitrogen ratio in ecosystems27,32,37. Regarding chitin degradation, the chitinase family includes three enzymes that act separately, (1) endochitinases that recognize o-glycosyl bonds between chito-saccharide residues for catalysis and produce multimers of oligosaccharides, especially diacetylchitobiose; (2) exochitinases that release soluble low molecular weight dimers, and (3) chitobiose that hydrolyses diacetylchitobiose to N-acetyl-glucosamine (GlcNAc)41. Both bacteria and fungi use chitin as a carbon and energy source, and the production of chitinolytic enzymes is related to carbon sources in synthetic culture media32,41.
Chitinases produced by Trichoderma correspond to enzymes that function as biological control agents and are responsible for the lysis and degradation of fungal cell walls and insect cuticles16. Furthermore, the exposure of Paenibacillus sp. to pyrethroids (cypermethrin) at concentrations recommended for field applications, caused the total inhibition of chitinase production, whereas insecticides such as methyl parathion and endosulfan significantly decreased (30–40%) the activity and stability of chitinases38. Organic molecules such as PAHs, with four or more aromatic rings, inhibited chitinase activities from Aeromonas hydrophila subsp. anaerogenes A52 and from T.harzianum29,41. To our knowledge, GlcNAc-activity has not been reported as being involved in the removal or degradation of organic pollutants such as pyrethroid and carbamate insecticides; however, this enzyme may serve as a biomarker for assessing detoxification processes of systems polluted with these compounds.
Endoglucanases (EGs) represent a group of dynamic cellulases that randomly attack internal O-glycosidic bonds of the cellulose chain, releasing glucan chains with different lengths, to generate new reducing and non-reducing ends; thus, EGs are the most important cellulases that contribute to the hydrolysis of cellulose50. EGs have many biotechnological applications for the industry, including animal food, textiles, laundry, pulp and paper, brewery and wine, food, and agriculture, among others. These enzymes are globally marketed from fungi such as Aspergillus and Trichoderma3.
Overall, most studies are limited to reporting the degradation percentages of insecticides but not emphasizing the effects of insecticides on microbial biomolecules. The production of a high number of enzymes, and the optimal activity of these enzymes may depend on culture media and microorganisms. The use of pyrethroids and carbamates increased as a result of the prohibition of DDT, and these insecticides can disturb agricultural soils and may lead to several issues related to environmental and human health pollution. Furthermore, these organic chemicals may exert toxic effects on microorganisms and higher organisms. The present study highlights that a commercial organic pesticide based on pyrethroids and carbamates may inhibit both the growth of filamentous fungi and the activity of important fungal enzymes involved in either chitin or cellulose degradation.
Overall, increasing the concentrations of the active ingredient of the commercial insecticide H24® decreased the radial growth rate in ten strains of Trichoderma sp. and P. chrysosporium. Four prominent strains of Trichoderma [T. koningii (Trich CP03), T. virens (Trich CP022), T. virens (Trich CP037), and T. atroviride (Trich CP0X)] were able to tolerate 200ppm of this commercial insecticide. These four fungal strains grew in a liquid culture medium contaminated with 100ppm of the commercial insecticide and showed increased protein production and high POX enzyme activity. Moreover, this commercial insecticide had negative effects on chitinase and endoglucanase activities derived from the Trichoderma consortium; therefore, it should be considered when using these microorganisms in combination with organic insecticides addressed to integrated pest management, and for assessing tolerance, detoxification, or degradation of organic insecticides based on permethrin, prallethrin and propoxur, for instance.
Conflict of interestThe authors declare that they have no conflicts of interest.
Authors thank CONAHCYT-Mexico for financial support provided to the postdoctoral position of CM-A at Colegio de Postgraduados. We thank the critical review of anonymous reviewers that contribute on improving this manuscript.