Antibiotic resistance results in higher medical costs, prolonged hospital stays and increased mortality and is rising to dangerously high levels in all parts of the world. Therefore, this study aims to search for new antimicrobial agents through bioprospecting of extracts of endophytic fungi from Bauhinia guianensis, a typical Amazonian plant used in combating infections. Seventeen (17) fungi were isolated and as result the methanolic extract of the fungus Exserohilum rostratum showed good activity against the bacteria tested. The polyketide monocerin was isolated by the chromatographic technique, identified by NMR and MS, showing broad antimicrobial spectrum.
La resistencia a los antibióticos conduce a mayores costos médicos, hospitalizaciones prolongadas e incremento de la mortalidad, y está aumentando a niveles peligrosamente altos en todas partes del mundo. Este estudio tuvo como objetivo la búsqueda de nuevos agentes antimicrobianos a través de la bioprospección de extractos de hongos endófitos de Bauhinia guianensis, una planta amazónica típica, utilizada en la lucha contra problemas infecciosos. Fueron aislados 17 hongos; el extracto metanólico del hongo Exserohilum rostratum mostró buena actividad contra las bacterias probadas. Se aisló monocerina policétido por la técnica de cromatografía; este compuesto fue identificado por RM y EM, y mostró un amplio espectro antimicrobiano.
Antibiotics are medicines used to prevent and treat bacterial infections. Antibiotic resistance occurs when bacteria change in response to the use of these medicines, resulting in higher medical costs, prolonged hospital stays and increased mortality and rising to dangerously high levels in all parts of the world. New resistance mechanisms emerge and spread globally every day, threatening our ability to treat common infectious diseases. A growing list of infections such as pneumonia, tuberculosis, blood poisoning and gonorrhea are becoming harder and sometimes impossible to treat as antibiotics become less effective14.
Currently there is growing interest in endophytic microorganisms, which, in association with their host, are known to produce a wide range of compounds with diverse biological activities3,4. There are several reports in the literature of compounds isolated from endophytic fungi having antimicrobial activity, such as the extract of fungus Nodulisporium sp. (Xylariaceae), isolated from the woody plant species Erica arborea (Ericaceae), from which nodule purine compounds D, E and F that act as antifungal, antibacterial and algicide were isolated7. Of the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides (Asteraceae) compounds 6-O-metilalaternina and altersolanol A were isolated, both having antimicrobial activity against gram-positive pathogens Staphylococcus aureus, Staphylococcus epidermidis and Enteorococcus faecalis2.
In the Brazilian Amazon it is common to use prepared plants to combat various diseases. Bauhinia guianensis is a typical Amazon plant popularly known as “ladder tortoise”, “liana toenail of the ox”, “nail of the cow” and “mororó liana”, which is used as a drug in folk medicine, specifically to fight infections, painful processes and diabetes. The phytochemical and pharmacological studies of this plant indicate that it produces glycosides, sterols, triterpenes, lactones and flavonoids1,10.
In a previous study different chemical constituents were isolated from B. guianensis, showing anti-inflammatory and analgesic activities5,13. It is known that some compounds of plant origin are produced by their endophytic fungi12 and that some of the biological activities described for plants can be found in their endophytes8.
Thus, the present work is aimed to perform the prospection of the antimicrobial activity of the extracts of endophytic fungi from B. guianensis to obtain antimicrobial compounds.
A specimen of B. guianensis (voucher specimen No. IAN 177,179) was collected from “Embrapa Amazônia Oriental – Belém, Brazil” and endophytic fungi were isolated after washing with water. A small fragment of plant was subjected to a series of immersions for disinfection and elimination of epiphytic fungi, first in hexane PA (Tedia®) for 1min, then in 70% ethyl alcohol for 30s, then in sodium hypochlorite solution 2% for 2min and finally in sterile water. After, the plant material was inoculated in Petri dishes containing PDA (Potato, Dextrose, Agar) culture medium for growth of colonies. Seventeen (17) fungi were isolated and purified by successive samplings. Fungi were identified by colony morphology and microscopic aspects observation on optical microscopy and DNA sequencing using the ITS4 region. Small fragments of the isolated fungi were transferred to 10ml flasks containing distilled water6 and stored in the mycology collection of the “Laboratorio de Bioensaios e Química de Micro-organismos/Universidade Federal do Pará” (LaBQuiM/UFPA).
Fungi were grown in two 125ml Erlenmeyer flasks containing 25g of rice and 10ml of distilled water each. The flasks were autoclaved at 121°C for 45min (Vertical Autoclave 75 L, Prismatec®), and then fungi were inoculated in sterile conditions and incubated at room temperature for 23 days in a static mode for growth of colonies. Then, methanol (Tedia®) was added to the biomass. After 24h, the material was filtered and the methanolic solutions were evaporated in a rotary evaporator (Quimis®, Q344B, São Paulo, Brazil) to obtain the extracts.
Susceptibility of the microorganisms to the test extracts was determined by the microbroth dilution assay as recommended by the Subcommittee on Antifungal Susceptibility Testing of the United State National Committee for Clinical Laboratory Standards9, which was performed on 96 well plates containing 100μl of Mueller Hinton Broth (MHB), 100μl of test extracts and 5μl of test bacteria at 1.0×108CFU/ml, followed by incubation at 37°C (24h). The test extracts obtained from the fungal culture were dissolved in dimethylsulfoxide at concentration 39–2500μg/ml. The test microorganisms were Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), S. aureus (ATCC 25923), Bacillus subtilis (ATCC 6633) and Salmonella Typhimurium (ATCC 14028) (provided by the Instituto Evandro Chagas – Belém, Brazil). Bioactivity was recorded as the absence of red color in the wells after the addition of 10μl of 2,3,5-triphenyltetrazolium chloride. Penicillin, vancomycin and tetracycline (25μg/ml each) were used as positive controls; the cultivation medium (MHB only) was used as negative control.
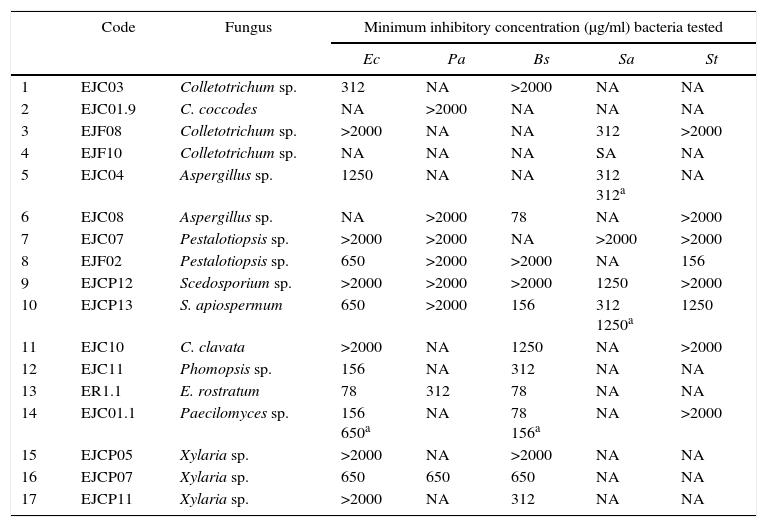
None of the extracts showed significant activity against bacteria P. aeruginosa and S. aureus. With respect to E. coli, the extract of the fungus Exserohilum rostratum (ER1.1) inhibited growth at a concentration of 78μg/ml and in the case of bacteria B. subtilis the greatest inhibitions were observed for the extracts of fungus Aspergillus sp. (EJC08), Paecilomyces sp. (EJC01.1) and E. rostratum (ER1.1) at a concentration of 78μg/ml. For S. Typhimurium bacteria the best result obtained was for the extracts of fungus Pestalotiopsis sp. (EJF02) at a concentration of 156μg/ml. Based on the results obtained (Table 1) the extract of fungus E. rostratum (ER1.1) was selected for the isolation of the active compound.
Results obtained to antimicrobial assay determined by microbroth dilution with methanolic extracts (2500–39μg/ml) of endophytic fungi from B. guianensis
| Code | Fungus | Minimum inhibitory concentration (μg/ml) bacteria tested | |||||
|---|---|---|---|---|---|---|---|
| Ec | Pa | Bs | Sa | St | |||
| 1 | EJC03 | Colletotrichum sp. | 312 | NA | >2000 | NA | NA |
| 2 | EJC01.9 | C. coccodes | NA | >2000 | NA | NA | NA |
| 3 | EJF08 | Colletotrichum sp. | >2000 | NA | NA | 312 | >2000 |
| 4 | EJF10 | Colletotrichum sp. | NA | NA | NA | SA | NA |
| 5 | EJC04 | Aspergillus sp. | 1250 | NA | NA | 312 312a | NA |
| 6 | EJC08 | Aspergillus sp. | NA | >2000 | 78 | NA | >2000 |
| 7 | EJC07 | Pestalotiopsis sp. | >2000 | >2000 | NA | >2000 | >2000 |
| 8 | EJF02 | Pestalotiopsis sp. | 650 | >2000 | >2000 | NA | 156 |
| 9 | EJCP12 | Scedosporium sp. | >2000 | >2000 | >2000 | 1250 | >2000 |
| 10 | EJCP13 | S. apiospermum | 650 | >2000 | 156 | 312 1250a | 1250 |
| 11 | EJC10 | C. clavata | >2000 | NA | 1250 | NA | >2000 |
| 12 | EJC11 | Phomopsis sp. | 156 | NA | 312 | NA | NA |
| 13 | ER1.1 | E. rostratum | 78 | 312 | 78 | NA | NA |
| 14 | EJC01.1 | Paecilomyces sp. | 156 650a | NA | 78 156a | NA | >2000 |
| 15 | EJCP05 | Xylaria sp. | >2000 | NA | >2000 | NA | NA |
| 16 | EJCP07 | Xylaria sp. | 650 | 650 | 650 | NA | NA |
| 17 | EJCP11 | Xylaria sp. | >2000 | NA | 312 | NA | NA |
Ec=E. coli, Pa=P. aeruginosa, Bs=B. subtilis, Sa=S. aureus, St=S. thiphymurium, NA=no action.
The methanolic extract obtained from selected E. rostratum fungi (ER 1.1) (2.0g) was fractioned on silica gel column chromatography using hexane/ethyl acetate (9:1, 4:1, 7:3, 1:1, 3:7), ethyl acetate, ethyl acetate/methanol (3:7, 1:1) and methanol resulting in 9 fractions. The fraction hexane/ethyl acetate 7:3 (230mg) was chromatographed on a silica gel column eluted with hexane/ethyl acetate (9:1, 4:1, 7:3, 1:1, 3:7), ethyl acetate resulting in 85 fractions pooled in F1 to F8; fraction F3 gave a solid orange-colored oil compound 1 (18mg). Compound 1 was identified by 1D and 2D nuclear magnetic resonance (NMR) and mass spectrometry (MS). After isolation and identification, compound 1 was tested against E. coli bacteria (ATCC 25922), P. aeruginosa (ATCC 27853), S. aureus (ATCC 25923), B. subtilis (ATCC 6633) and S. Typhimurium (ATCC 14028) at a concentration of 500–7.8μg/ml.
The 1H and 13C NMR experiments were recorded in a NMR spectrometer (Mercury 300, Varian, Oxford, Oxfordshire, UK) with CDCl3 (Cambridge®) as solvent and standard. MS spectra were carried out in the mass spectrometer (Acquity TQD, Waters, Milford, MA, USA) using electrospray ionization in positive ion mode, ESI(+). The specific rotation was performed using specific rotation equipment (Nova Instruments NO 1412, Piracicaba, Brazil).
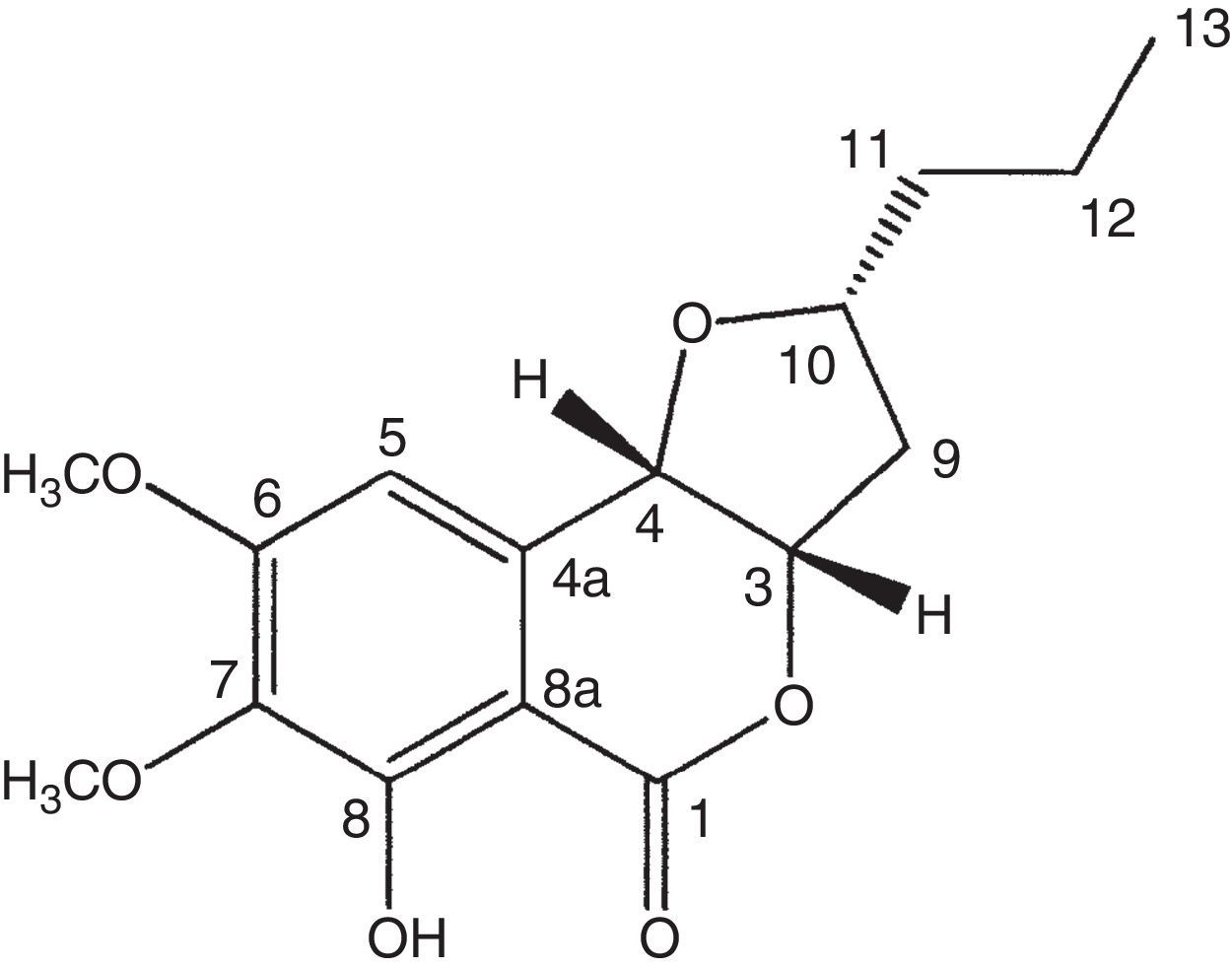
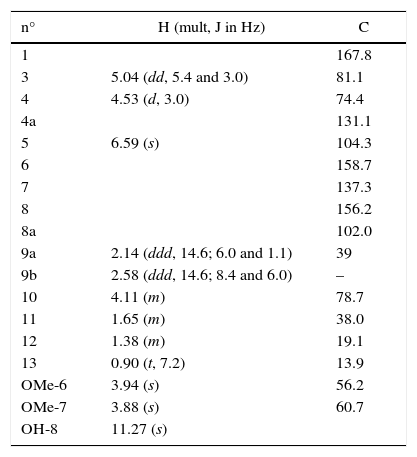
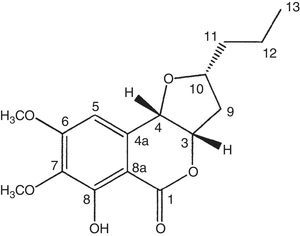
Compound 1 was isolated as orange oil and was optically active with the specific rotation [α]D: +60 (c 1.0, dichloromethane). The molecular formula C16H20O6 was calculated as base of its mass spectrum ESI(+) m/z 309 [M+H]+. The 1H NMR spectrum showed signal to the chelated hydroxyl (δH 11.27, OH-8) and signals to an aromatic ring hydrogen (δH 6.59), three carbinolic hydrogens (δH 5.04, 4.53 and 4.11), and two OCH3 groups (δH 3.94 and 3.88). The analysis of the 13C NMR and HSQC spectra showed the presence of a carbonyl ester conjugate (δC 167.8) chelated by hydrogen bond with hydroxyl, six carbons of the aromatic ring, three of which were are oxygenated (δC 158.7, 156.2 and 137.3) and one unreplaced (δC 104.3), three carbinolic carbons (δC 81.1, 78.7 and 74.4), three methylene (δC 39.0, 38.0 and 19.1), and methyl (δC 13.9). NMR data to compound 1 (Table 2) were consistent with the structure of the isocoumarin derivative, monocerin15 (Fig. 1).
1H and 13C NMR data (300MHz and 75MHz, CDCl3) to compound 1
| n° | H (mult, J in Hz) | C |
|---|---|---|
| 1 | 167.8 | |
| 3 | 5.04 (dd, 5.4 and 3.0) | 81.1 |
| 4 | 4.53 (d, 3.0) | 74.4 |
| 4a | 131.1 | |
| 5 | 6.59 (s) | 104.3 |
| 6 | 158.7 | |
| 7 | 137.3 | |
| 8 | 156.2 | |
| 8a | 102.0 | |
| 9a | 2.14 (ddd, 14.6; 6.0 and 1.1) | 39 |
| 9b | 2.58 (ddd, 14.6; 8.4 and 6.0) | – |
| 10 | 4.11 (m) | 78.7 |
| 11 | 1.65 (m) | 38.0 |
| 12 | 1.38 (m) | 19.1 |
| 13 | 0.90 (t, 7.2) | 13.9 |
| OMe-6 | 3.94 (s) | 56.2 |
| OMe-7 | 3.88 (s) | 60.7 |
| OH-8 | 11.27 (s) |
Antimicrobial testing was performed for compound 1, showing good activity against E. coli bacteria (MIC 15.62μg/ml and MBC 250μg/ml), P. aeruginosa (MIC 15.62μg/ml), B. subtilis (MIC 15.62μg/ml and MBC 62.5μg/ml), S. aureus (MIC 62.5μg/ml) and S. Typhimurium (MIC 31.25μg/ml). Data suggest that compound 1 is responsible for the activity observed for the E. rostratum extract. Monocerin has been isolated as an antifungal, insecticidal, and phytotoxic secondary metabolite from several fungal species including Helminthosporium monoceras, Exserohilum turcicum, Fusarium larvarum and Microdochium bolleyi and showed to be also active against Plasmodium falciparum11,15. Zhang et al. (2008)15 isolated monocerin from fungus strain M. bolleyi and presented antimicrobial activity against E. coli in the agar diffusion test.
The present work isolated 17 endophytic fungi from B. guianensis, being the methanolic extract of fungus E. rostratum the most active. Monocerin was isolated by chromatographic procedures, showing good antimicrobial activity. This work reports for the first time the isolation of monocerin from endophytic fungi of this Amazon plant, highlighting the activity of this compound as a broad spectrum antimicrobial agent.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors thank Fundação Amazônia de Amparo a Estudos e Pesquisa do Pará (FAPESPA), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) for their financial support.