Candida albicans is a commensal of the mammalian microbiome and the primary pathogenic fungus of humans. It becomes a severe health problem in immunocompromised patients and can cause a wide variety of mucosal and systemic infections. The interaction between C. albicans and host cells is characterized by the expression of virulence factors such as adhesins and invasins, the secretion of hydrolytic enzymes, a transition from yeast to filamentous hyphae form, and the ability to form biofilms; these features collectively result in cell adhesion, invasion, and damage. This review describes complex commensal interactions of C. albicans with host cells and the cellular events that it triggers in a pathogenic environment. We also review the host immune response induced by C. albicans antigens and the mechanisms developed by this fungus to avoid the action of antifungal agents.
Candida albicans es un comensal del microbioma de mamíferos y el principal hongo patógeno de humanos. En pacientes inmunocomprometidos se convierte en un grave problema de salud por causar una amplia variedad de infecciones en mucosas y sistémicas. La interacción entre C. albicans y las células del huésped lleva a la expresión de factores de virulencia, como adhesinas e invasinas, a la secreción de enzimas hidrolíticas y a la transición de levadura a hifa filamentosa, capaz de para formar biopelículas, lo que genera adherencia, invasión y daño celular. En esta revisión describimos la compleja interacción comensal de C. albicans con la célula huésped y los eventos celulares que ejecuta en un ambiente patogénico. También se revisa la respuesta inmunitaria del huésped inducida por antígenos de C. albicans y los mecanismos desarrollados por este hongo para evitar la acción de agentes antifúngicos.
Fungal infections cause about 1.7 million deaths worldwide per year mainly in immunocompromised individuals with two or more pathological conditions6,42. The incidence of infections caused by the genus Candida has steadily increased since the 1970s perhaps due to an increased risk of opportunistic infections, the improvement in clinical procedures that identify fungi causing nosocomial infections, as well as the development of antifungal resistance due to prolonged exposure to treatment11.
There are currently more than 150 species of Candida, and approximately 20 are known to cause infections in humans. Candida albicans is the main causative agent of candidiasis and the primary fungal infection in adults and pediatric patients8,66,82. In the USA, it was reported that sepsis caused by C. albicans has a mortality rate of approximately 40%, which is higher than any other sepsis caused by bacteria or fungi89. Infections caused by the genus Candida are the main cause of nosocomial fungal infections especially in tertiary care hospitals17.
In recent decades, other non-albicans species such as C. glabrata, C. parapsilosis, C. tropicalis, C. krusei (Pichia kudriavzevii), C. dubliniensis, C. kefyr, C. famata, C. auris and others have become highly relevant at the clinical level due to their increasing prevalence as etiologic agents of candidiasis20,49,73,79,82. In particular, C. auris is considered an emerging serious global health threat by the Centers for Disease and Control Prevention (CDC) because of its multidrug resistance. However, C. albicans stands as the major fungal pathogen of humans8,82.
Candida albicansC. albicans has various yeast-like morphologies (white, opaque, gray, and intestinal), two forms of hyphae (linear and sinusoidal), a pseudohyphae, and chlamydospores (Figs. 1 and 2). Pseudohyphae remain attached after cytokinesis and generate mycelia after multiple rounds of cell division similar to hyphal cells. In addition to this great diversity of forms, C. albicans can grow in single-cell cultures, biofilms, and microcolonies15.
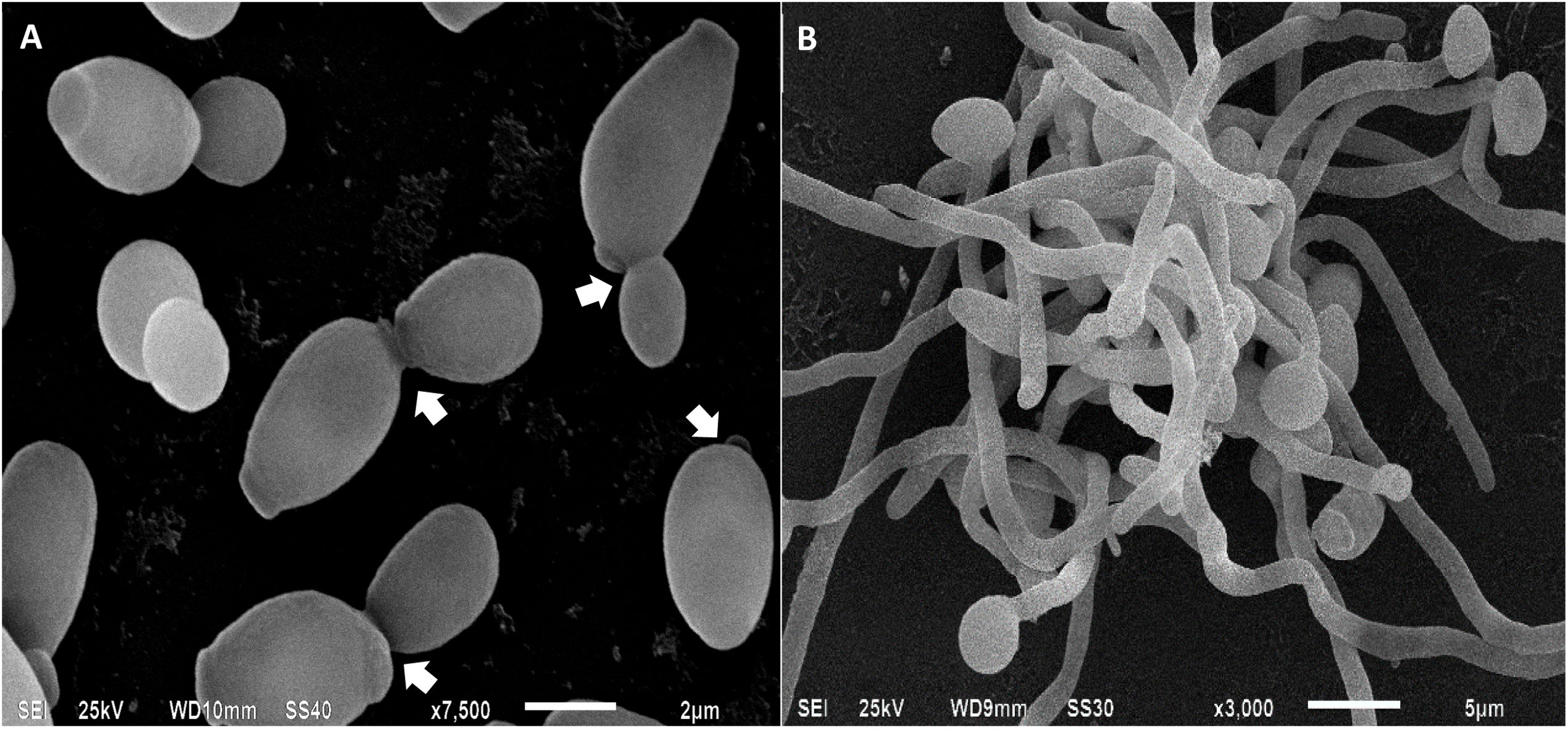
Candida albicans cells analyzed by scanning electron microscopy. In (A), yeasts are in the process of budding, the arrows indicate the site of cell division between the mother cell and the daughter cell. In (B), mycelia of C. albicans, involved in tissue invasion during the infectious process.
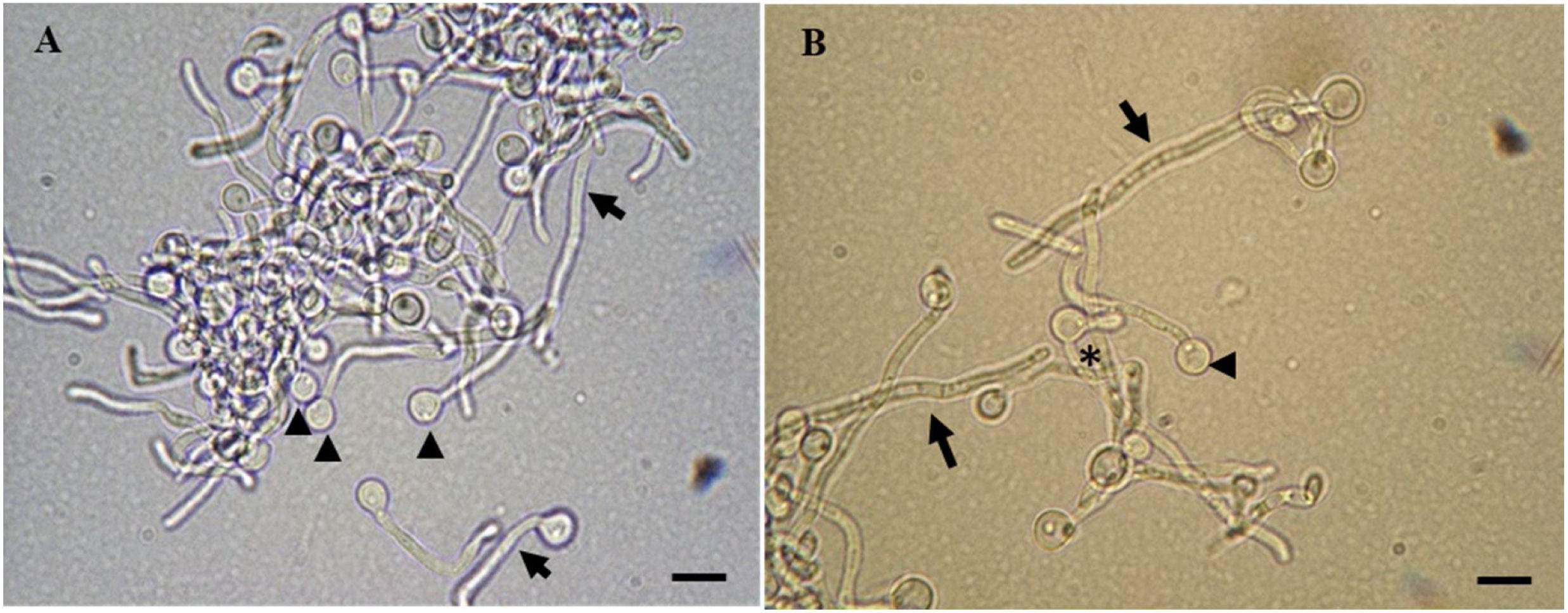
C. albicans cells in cell transition analyzed by light microscopy. In (A), a combination of chlamydospores (arrowheads) and hyphae (arrows) are found. In (B), pseudohyphae (asterisk), chlamydospores (arrowhead), and multicellular hyphae (arrows) are seen. Scale bars represent 5μm.
As a commensal fungus, C. albicans asymptomatically colonizes the oral mucosa, skin, vagina, and gastrointestinal tract of healthy individuals, and represents over 80% of vaginal and oral yeast strains isolated from asymptomatic humans. Therefore, it has various characteristics, both commensal and virulent, which allows it to be part of the natural microbiome in humans and, to invade tissues and organs15,85,89 in the case of a weakened immune system.
C. albicans has a complex interaction with the host through the cell wall, which is the main structure in contact with the host. It is essential for fungal cellular integrity. The fungal cell wall protects the cells from environmental stress, including drastic changes in temperature, osmotic changes, dehydration, and immune response9.
Among the most critical components of the C. albicans cell wall are chitin, glucan, and mannoproteins9. The latter are vital in morphogenesis and pathogenesis because they function as environmental sensors, structural elements, cross-linking enzymes, and adhesins; these components could be the point of interest in generating new antifungal drugs. Most of the proteins are characterized by their high heterogeneity, low abundance, low solubility, and hydrophobicity72. The recent analysis of the proteome of the C. albicans cell wall by mass spectrometry demonstrated the presence of proteins S7A, S13, and S16A of the 40S ribosome as well as the 60S ribosome protein L30, the glycolytic enzymes enolase 1 (Eno1), glyceraldehyde-3 phosphate dehydrogenases 1 and 2, pyruvate kinase, fructose bisphosphate aldolase, phosphoglycerate kinase 1 (Pgk1), phosphoglycerate mutase, and pyruvate decarboxylase, heat shock proteins SSA1 and SSB1, mitochondrial outer membrane protein porin 1 and ADP/ATP transporter protein, the plasma membrane ATPase PMA1 and the galactose transporter-related protein Hgt7, the elongation factor 1-α 1, cell wall agglutinin-like sequence protein 1 (Als1) and 2 (Als2), and the proteins 1,3-β-glucanosyltransferase PGA4 and chitinase 274. Of these proteins, Eno1, Pgk1, and Als1 showed favorable immunogenicity21,77. Experimental evidence indicates that Eno1 has transglutaminase activity in the cell wall of C. albicans, which is of crucial importance in osmotic protection and transition from yeast to mycelium75. Eno1 is the main antigen in patients with candidiasis39. Leu et al.47 demonstrated that previous immunization with anti-Eno1 (CaS1) antibodies prolonged the survival of mice and zebrafish that developed candidiasis. CaS1-treated mice had significantly reduced levels of inflammatory cytokines and lower fungal load in the kidney and spleen. The authors also indicated that anti-Eno1 (CaS1) antibodies could be a potential immunotherapeutic treatment against C. albicans infections47.
When in contact with the host, epithelial cells are the first line of defense: They induce the expression of antimicrobial peptides such as defensins, cathelicidins, and histatins, which favor the control of C. albicans growth in the commensal phase and during infection36.
In addition, the tight junctions seal the space between the host cell surface and the mucosal lamina propia, thus preventing interepithelial invasion by C. albicans97. In the case of intestinal or vaginal epithelial cells, the mucus layer composed of mucins promotes protection and prevents direct contact with the epithelium. In the oral cavity, saliva contains various antimicrobial agents such as lactoferrin, lysozyme, histatins, cathelicidin (LL-37), calprotectin, and defensins, forming a chemical barrier that prevents the growth of C. albicans36,97. However, patients in intensive care surgical units receive prolonged treatment with antibiotics, chemotherapy, or immunosuppressive drugs that favor the conversion of the yeast C. albicans into pathogenic cells (hyphae and pseudohyphae), thus leading to candidiasis8,82. Therefore, C. albicans changes into an opportunistic fungus when there is an imbalance between the host's immunity and the commensal microenvironment, thus causing superficial or disseminated infection18,32.
Virulence factors contributing to the pathogenesis of C. albicansCandida albicans has various virulence factors such as the morphological transition from yeast cell to hyphae (both forms seem to be involved in pathogenesis), the expression of adhesins and invasins on the cell surface, the ability to damage host cells, biofilm formation, and secretion of hydrolytic enzymes89.
When in contact with host cells, the cell wall of C. albicans has a role in adhesion to host cells through adhesins as well as intercellular communication, i.e., the “glycan code.” This interaction results in the development of a pathogenic state or the production of a resistance reaction by the host70. The central regulator of fungal adherence is the transcription factor Bcr1 whose target genes are ALS3, ALS1, ECE1, and HWP1 that also favor biofilm formation62. Among the most studied adhesins are agglutinin-like sequence proteins (ALS). Of the ALS family proteins (Als1-9), Als 5-7 and 9 are found on the surface of yeast cells. Als3 is expressed exclusively in the hyphae and binds E-cadherins on epithelial cells and N-cadherins on endothelial cells. Als3 and Als1 are involved in C. albicans biofilm formation. Hosseini et al.37 reported clinical isolates of C. albicans expressing Als1 and Als3 that were resistant to fluconazole; adherence was more substantial than in the controls (cells that do not express Als1 and Als3).
Another protein capable of binding epithelial E-cadherins is Ssa1 (Hsp70-like heat shock protein). Als3 and Ssa1 induce endocytosis of C. albicans in host cells. However, the active penetration generated by hyphae is the primary mechanism implemented by C. albicans to invade tissue57,59.
Another crucial virulence protein is hyphal wall protein 1 (Hwp1): The N-terminal region of Hwp1 serves as a substrate for epithelial cell transglutaminase, thus resulting in covalent binding of C. albicans to epithelial cell proteins, which appears to be essential in the development of oropharyngeal candidiasis but not for disseminated candidiasis. The adhesion of Candida cells by the action of the mentioned adhesins can induce endocytosis and active penetration into the host cells to later spread in the tissues, bloodstream, and organs52.
Pleomorphic transitionThe morphological change from unicellular yeast to filamentous hyphae form is crucial in the virulence of C. albicans. The virulence capacity of hyphae is not produced by their morphology per se but by the expression of specific genes. For example, the Csa2 protein participates in the utilization of iron from hemoglobin and favors the growth of hyphae38. Another protein involved in the regulation of morphogenesis and biofilm formation is Hgc, which is related to cyclin G1 and the deletion of the gene encoding HGC1 attenuates the virulence of C. albicans in systemic infections in mice98.
Various studies indicate that in an infectious process, the C. albicans yeast cells spread to other regions of the organism via a phagocyte-dependent mechanism, where neutrophils and macrophages can be vehicles for dissemination, while the hypha invades and damages host tissue by directing growth in response to contact with a solid surface, a process called thigmotropism28,36.
Macrophages phagocytize fungal cells in animal models of C. albicans infection. Hypha formation within the macrophages is related to damage to the phagosome. This process induces metabolic starvation and the evasion of the acidic environment by changing the pH value to neutral due to the production of neutralizing metabolites44. Hyphal formation and β-glucan masking inhibit phagosome acidification of macrophages, thus leading to intracellular hyphal formation and causing macrophage cell death due to physical forces3. The binding of Staphylococcus aureus cells to the hyphae of C. albicans facilitates the dissemination of bacteria through mucosal barriers. These are often isolated simultaneously in cases of infections associated with biofilms38.
Candida albicans biofilmsMicrobial biofilms are communities of cells that adhere to solid surfaces or are present at liquid–air interfaces. They are the most common growth stage of growth for many microbial species and are often more resistant to drugs and physical insults51. The cellular composition of C. albicans biofilms includes two main types of cells: yeast (small, oval cells) and long, tubular hyphal cells27.
As the structure of C. albicans biofilm matures, its outer layers release yeast cells, which are thought to disperse and mediate the spread of C. albicans to other tissues; therefore, they are an essential part of the infection process95. Various studies show that the Candida biofilm matrix comprises carbohydrates, proteins, nucleic acids, hexosamine, phosphorus, and uronic acid2. This matrix controls cell dispersion, protects against the immune system, and participates in developing resistance to azoles, polyenes, and pyrimidine analogs by not allowing the passage of these antimycotics81. One of the main components of the extracellular biofilm matrix is β-1,3 glucan. Different genes (BGL2, PHR1, and XOG1) are involved in crosslinking during planktonic cell growth, remodeling, and elongation of glucan chains88. The extracellular polysaccharide matrix of Candida biofilms facilitates adhesion to surfaces and other biopolymers, thus generating biofilms with structural integrity33. C. albicans has two transcriptional regulators of extracellular matrix generation in biofilms: Rlm1 and Zap1. Experimental evidence indicates that deletion of Rlm1 decreases matrix levels. Conversely, Zap1 deletion leads to the accumulation of excess extracellular matrix63.
The life cycle of C. albicans biofilms begins when planktonic yeast cells adhere to the substrate, i.e., either hard (such as biomaterials that are part of a prosthetic device or the surface of a denture) or soft (such as a layer of mucosal epithelium in the oral or vaginal cavity) to then proliferate and mature into a structured biofilm composed of layers of yeast, pseudohyphae, and hyphae. The cells that make up the biofilm then disperse or their disintegration occurs at the end of the cycle31,92.
One of the main problems in hospital environments is the formation of biofilms on the surface of medical devices implemented in patients’ treatment. For example, the formation of biofilms on catheters, heart valves and dentures have a high capacity to promote infections that progressively spread to the bloodstream and generate systemic infections50,63. Current estimates of the biofilm development risk on catheters is 30%; however, this depends on the location of the device53.
Biofilms are characterized by drug resistance, and thus, removing the device that exhibits biofilm formation ends up being the most viable and effective option to reduce the risk of systemic infection. However, it is problematic if the patients are in a severe state of the disease or need a surgical procedure for their removal50.
Secreted hydrolytic enzymesVarious hydrolytic enzymes (proteases, phospholipases, lipases, and hemolysins) secreted by C. albicans contribute to the host invasion process. Secreted aspartic proteases (Saps) encoded by a family of genes (SAP1 to SAP10) are factors associated with the virulence of C. albicans. They degrade human proteins such as hemoglobin, albumin, keratin, collagen, laminin, fibronectin, mucin, and almost all immunoglobulins. Sap9 and Sap10 remain bound to the cell surface and are strongly involved in biofilm formation by C. albicans unlike other members of the family41. However, experimental evidence suggests that Sap1 to Sap6 do not play a significant role in the virulence of C. albicans in a murine model of disseminated candidiasis13.
Phospholipases (PLs) play an essential role in tissue invasion by Candida. PLs are enzymes that hydrolyze one or more ester bonds in glycerophospholipids. Studies have evaluated the importance of phospholipase B (PLB) in pathogenicity via the disruption of the host membranes; they allow the tip of the hypha to enter the cytoplasm, thus indicating that the null mutant of the same (PLB−/−) exhibited attenuated virulence in animal models65. Other PLs including PLC1, PLC2, and PLC3 subtypes are involved in the virulence of C. albicans because the heterozygous plc1/PLC1 mutant and the mutants lacking PLC2 and PLC3 were deficient in hyphae formation68.
Lipases are another class of lipolytic enzymes that contribute to the virulence of C. albicans. These enzymes catalyze the hydrolysis of the ester bonds of triglycerides, thus releasing fatty acids. Paraje et al.67 demonstrated in vitro that the lipase released from C. albicans induces cytotoxicity and favors the accumulation of lipid droplets in the cytoplasm of macrophages and hepatocytes67. During systemic infection, C. albicans produces hemolysins that lyse erythrocytes to obtain iron—an essential element for the growth and metabolism of this fungus. A comparative analysis of the ability of C. albicans and non-albicans Candida isolates from the oral cavity of human immunodeficiency virus (HIV)-positive patients showed that 92% of the species produce hemolysins with 58% of the strains exhibiting intense hemolytic activity78.
Another virulence factor of C. albicans is the cytolytic exotoxin “candidalysin” that is only produced by the hyphae. It is essential during mucosal and systemic infection58,61. Candidalysin is an amphipathic molecule that takes on an α-helical structure and facilitates the permeability of host cell membranes, thus resulting in the efflux of lactate dehydrogenase protein and destabilizing the host cell membranes86. The host may recognize C. albicans as a potentially pathogenic agent and initiate an immune response at this stage. Candidalysin is an immunostimulatory molecule and favors the influx of cytokines into the epithelium when C. albicans infection occurs35.
In the oral infection, epithelial cells respond directly to the presence of candidalysin due to the activation of the epidermal growth factor receptor (EGFR), which is essential in the infection process35. This activation triggers the mitogen-activated protein kinase (MAPK) signaling pathway, which comprises ERK1/2 (extracellular signal-regulated kinase 1/2), JNK (c-Jun N-terminal kinase), and p3861. This leads to the production of interleukins 1 and 36 (IL-1 and IL-36) to promote the proliferation of innate TCR+ T cells as well as the expression of IL-17A, which is essential for antifungal defense in the oral mucosa94. Candidalysin can also induce the production of IL-1β, IL-36, and the NLRP3 inflammasome proinflammatory substances that help IL-17-mediated diseases34.
Immune responseThe first step in the host defense against C. albicans is the innate immune system—particularly neutrophils, dendritic cells, and macrophages56. These phagocytes detect pathogen-associated molecular patterns (PMAPs) through pattern recognition receptors (PRRs), thus resulting in signal-mediated transcription and subsequent secretion of inflammatory mediators such as chemokines and cytokines that recruit other immune cells to eliminate the pathogen at the site of infection and activate the adaptive immune response60.
C. albicans PMAPs responsible for the inflammatory response reside in the outer and inner layers of the cell wall23. The main PAMPs that trigger cytokine production and phagocytosis are mannoses and glucan, which are recognized by a variety of receptors60. The main PRR groups that recognize C. albicans are C-type lectin receptors, RIG I-type receptors, NOD-type receptors, and “Toll”-type receptors5. In the case of candidalysin, an activation of EGFR signaling is induced in a PAMP-independent manner promoting the secretion of neutrophil-targeted chemokines35.
As a consequence of the hostile action of C. albicans, the epithelium in which the infection is found will tend to induce an innate immune response through the release of alarmins (also called damage-associated molecular patterns, DAMP), adenosine monophosphate (AMP), and chemokines of immune cells. The alarmins may be recognized by the receptors of the innate immune cells with subsequent activation16,34.
In the case of secondary infection by C. albicans, a synergy occurs between the activation of phagocytic cells through dectin-1/CARF9 or Toll 2 receptor-like pathways with T-helper 17 (Th17) lymphocytes that recognize Candida48. Individuals with mutations in genes that disrupt the T-helper 17 cells (Th17) or interleukin-17 receptor (IL-17R) signaling pathway are highly capable of becoming infected and developing chronic mucocutaneous candidiasis93.
High-risk people currently receive antifungal prophylaxis. Although it effectively prevents Candida infections, its prolonged use can lead to the development of strains resistant to available antifungals22.
Clinical aspectsC. albicans is believed to enter the gastrointestinal tract and colonize human skin as it passes through the birth canal during childbirth96. In healthy people, the presence of this microorganism is benign; however, immunosuppressed patients can frequently suffer from infections of the oral cavity called “oral candidiasis” (OC). Immunosuppressive infections such as HIV are a significant risk factor for developing OC in addition to the use of dentures and being very young or very old54. Pseudomembranous candidiasis is the classic presentation of OC. It manifests as white plaques that can be found on the tongue, buccal mucosa, oral pharynx and soft and hard palates. These lesions are caused by yeast overgrowth on the oral mucosa with desquamation of epithelial cells and accumulation of fungal hyphae, keratin, fibrin, and necrotic tissue55.
Another type of candidiasis that affects millions of women each year is vulvovaginal candidiasis (VVC). It is estimated that approximately 75% of all women suffer from VVC at least once in their lives85. Predisposing factors for developing VVC are less well-defined than for OC and include uncontrolled diabetes mellitus, use of antibiotics, oral contraception, pregnancy, hormonal therapy, immunosuppression, corticosteroids, and genetic predisposition29. The main symptoms of VVC include itching, burning, and pain in the vaginal and vulvar tissue; these are commonly accompanied by odorless vaginal discharge85. Recent evidence shows that VVC is an immunopathology in which the immune response specifically involving the patient's neutrophils and related cytokines exacerbates the symptoms of the disease without adequately controlling the growth of Candida76.
A complication caused by C. albicans is systemic candidiasis; its dissemination begins with the invasion of mucosal surfaces and subsequent entry into the bloodstream87. This infection is difficult to diagnose because the symptoms are similar to systemic bacterial infections. Systemic candidiasis is associated with a high mortality rate, neutropenia, and damage to the gastrointestinal mucosa54. Other factors that promote the development of disseminated candidiasis include central venous catheters that allow direct access of the fungus to the bloodstream, the application of broad-spectrum antibiotics (that favor antifungal resistance), and disrupted mucosal barriers including gastrointestinal barriers during trauma or surgery54. During systemic candidiasis, C. albicans has to break through the endothelial lining of blood vessels at the site of infection. Likewise, once disseminated, it must go back through the endothelium of the blood vessels to leave the circulation30. When C. albicans leaves the circulatory system, it spreads to virtually any organ including the brain, kidney, liver, and lung often leading to death43.
Infection by SARS-CoV-2 (a type of respiratory coronavirus) causes COVID-19. This is mainly transmitted by respiratory droplets through close contact between people12. Between 5 and 30% of patients with COVID-19 develop a critical illness and require admission to the ICU where they may undergo mechanical ventilation, parenteral nutrition, broad-spectrum antibacterial therapy, indwelling central venous catheters, and administration of corticosteroids (due to their immunosuppressive capacity). These conditions favor the development of secondary infections69. COVID-19 is not a cause of—nor does it imply a direct association with—infection caused by C. albicans. This is because the immune system cells, which are the most important in the defense against C. albicans are not committed, e.g., monocytes, macrophages, and neutrophils. However, the use of indwelling central venous catheters (because of their propensity to form biofilms) and the administration of corticosteroids in patients with COVID-19 present a risk of producing a nosocomial infection caused by C. albicans. In fact, it has become a major concern because the transition from superficial to invasive candidiasis produces a lethality rate of 70% in patients with COVID-19 in critical condition19,24. In a recent report of 989 Italian individuals with COVID-19, 21 candidemia cases were detected, indicating a marked prevalence among these patients versus a historical cohort64.
Drugs used to treat infections caused by C. albicansFungal resistance to currently used drugs is a growing problem, and although C. albicans is the most common species of Candida that causes severe infections, other species such as C. auris, C. glabrata and C. parapsilosis8 have developed vast antifungal resistance. The antifungals mainly used to treat infections caused by Candida species are the azoles, echinocandins, and polyenes; however, these drugs tend to not be successful when the infection involves the C. albicans biofilm14,26. Unfortunately, these treatment options have become unsatisfactory due to the increased development of resistance, selective pressure, the unavailability of conventional antifungals for systemic administration, and adverse effects at higher drug concentrations40.
Resistance to antifungal drugs can be divided into two forms: clinical and mycological resistance. Clinical resistance is the incomplete eradication of the fungus in a patient who was administered a drug with antifungal activity in vitro against the fungus in question. Mycological resistance allows the fungus to grow and reproduce despite the presence of the drug with antifungal activity demonstrated in vitro71. Antifungals currently used to treat candidiasis have certain deficiencies, for example, polyenes that cause nephrotoxicity, echinocandins with an exclusive intravenous route, and some thiazoles that have toxicity and difficulties in their absorption45.
AzolesAzoles are responsible for inhibiting the growth and replication of fungi by inhibiting the enzyme lanosterol 14-α-demethylase (Erg11p). This enzyme is responsible for the conversion of lanosterol into ergosterol—a limiting step in the biosynthesis of ergosterol, which is the most abundant fungal cell membrane7. Fluconazole, itraconazole, and voriconazole are azole drugs that are used to treat C. albicans infections due to their high bioavailability25. Fluconazole is the most widely used antifungal drug; however, the current antifungal resistance exhibited by many fungal species has caused a limitation in its use90.
EchinocandinsEchinocandins are the drugs of choice for most cases of candidemia and invasive candidiasis80. The mechanism of action of echinocandins is the non-competitive inhibition of (1,3)-β-d-glucan synthase—this molecule synthesizes the polysaccharide 1,3-β-d-glucan, which is part of the cell wall. It causes osmotic deregulation of the fungus and subsequent lysis. There are currently three molecules that work under this concept: caspofungin, micafungin, and anidulafungin46,91. Resistance to these drugs is developed thanks to mutations in the FKS gene that encodes the enzyme glucan synthase4.
PolyenesFungicidal polyenes are amphipathic drugs. The main mechanism of action of these chemicals is their binding to the fungal cell membrane ergosterol to form transmembrane channels, thus acting as an exit gate for the cell contents (including K+ and Na+ ions) until the cell lyses83,84. However, due to the similarity between ergosterol and cholesterol and the low solubility of polyenes, the latter compounds tend to have substantial adverse effects in patients, leading to further limiting their use. Some examples of polyenes are amphotericin B and nystatin10.
Resistance to polyenes is due to changes in ergosterol molecules or plasma membrane content as well as the exchange between ergosterol, cholesterol, or stigmasterol (components of the cell membrane) for 3-hydroxy or 3-oxo sterols, which have less affinity for polyenes1.
ConclusionsThere has been a significant increase in potentially fatal infections caused by Candida. As the main pathogenic fungus of humans, C. albicans is characterized by a complex interaction with host cells, the bacterial microbiome, and the immune system. The increase in people facing predisposing conditions such as cancer chemotherapy, organ transplants, or HIV infections is becoming a greater risk for developing yeast infections. These infections could be managed more effectively if more rapid and specific diagnostic and therapeutic alternatives were available as well as the development of new therapeutic alternatives for the timely identification of fungal species and species with multi-drug resistance in patients undergoing long-term therapies. Therefore, understanding how the interactions of virulence factors, the microbiome, and the host response contribute to a Candida infection is of clinical relevance. There is also a need for laboratories to perform routine in vitro susceptibility testing in isolates from immunocompromised patients.
Conflict of interestsThe authors declare no conflict of interest.
We thank the Center for Research and Advanced Studies of the National Polytechnic Institute for providing us with the facilities to perform optical and scanning electron microscopy, and the Faculty of Medicine “Dr. Alberto Romo Caballero” of the Autonomous University of Tamaulipas for providing us with their facilities and to Conacyt for the doctoral scholarship granted with the number 346900 with which the experiments were carried out.