Fungi from the genus Cladorrhinum (Ascomycota) are promising agents in the biocontrol of phytopathogens, in the promotion of plant growth, and in the production of enzymes with technological application. We analyzed comparatively the ability of 5 native strains of Cladorrhinum samala and Cladorrhinum bulbillosum with reference strains belonging to the same genus. We used 95 individual carbon sources available in microplates from the Biolog® FF system. Although most of the strains mainly used soluble carbohydrates, the metabolic profile was highly dependent upon each isolate and it revealed intraspecific physiological variability in Cladorrhinum species.
Los hongos del género Cladorrhinum (Ascomycota) son agentes prometedores en el biocontrol de fitopatógenos, la promoción del crecimiento de las plantas y la producción de enzimas con aplicación tecnológica. En este trabajo se analizaron comparativamente las habilidades de 5 cepas nativas pertenecientes a las especies Cladorrhinum samala y Cladorrhinum bulbillosum con cepas de referencia del mismo género. Se usaron 95 fuentes individuales de carbono, disponibles en microplacas de Biolog® FF system. Aunque la mayoría de las cepas utilizaron principalmente carbohidratos solubles, el perfil metabólico fue altamente dependiente de cada aislamiento y reveló variabilidad fisiológica intraespecífica en las especies de Cladorrhinum.
The genus Cladorrhinum Sacc. and Marchal (Lasiosphaeriaceae, Sordariomycetes, Ascomycota [IndexFungorum; http://www.indexfungorum.org/names/Names.asp]) includes a fungal group of fundamental importance for agriculture and livestock, because some species have potential as agents in the biocontrol of fungal phytopathogens, in the promotion of plant growth, and in the production of phytases (U.S. Patent No. 6,514,495 from strain Cladorrhinum foecundissimum CBS 427.97)16. This genus includes representatives with a diagnostic conidial system that can be found in roots as endophytes or as saprotrophic forms on dung, soil or plant material, and is considered an ammonia fungus17. However, some species have also been associated with human and animal opportunistic diseases6.
Today the use of microbial-based fertilizers has gained significance in the effort to reduce the negative environmental effects generated by the excessive and/or improper application of chemical fertilizers. Although some Cladorrhinum strains have been proposed as promising agents in the development of biofertilizers for plant production, the knowledge of the nutritional features of these fungi, which are important in the industrial manufacturing of new biofertilizers using them, is scarce7. Carmarán et al.3 reported data about the growth of three strains in a standard agar medium under a narrow range of temperature. However, analysis of nutritional preferences based on carbon substrate utilization profiles can be used to identify and characterize phenotypical diversification in Cladorrhinum strains and to characterize the Biolog FF MicroPlates carbon compounds for fungal growth.
The aim of this work was to characterize 10 strains from the genus Cladorrhinum through carbon-substrate utilization profiles by the Biolog® system (Biolog Inc., Hayward, CA) and evaluate the physiological behavior of the strains related to the taxonomic delimitation of the species of the genus by comparison with the type strains.
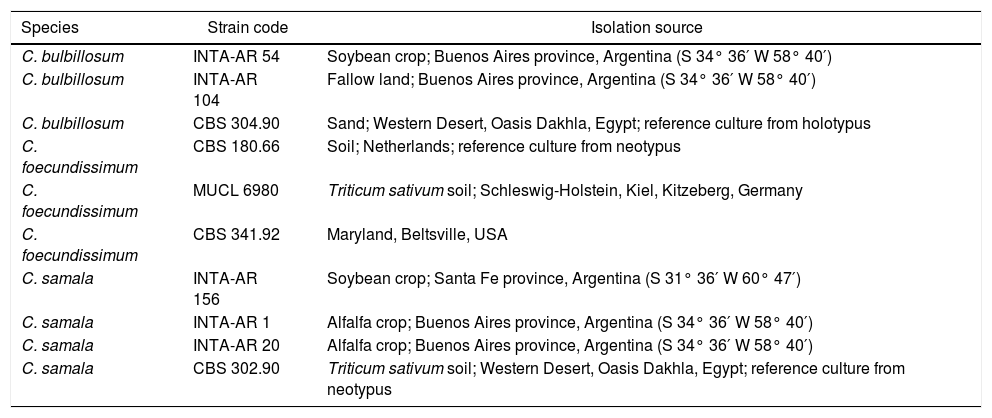
In this study we used 5 reference strains from Cladorrhinum samala, Cladorrhinumbulbillosum and Cladorrhinum foecundissimum and 5 native strains corresponding to Cladorrhinum samala and Cladorrhinum bulbillosum deposited in the fungal collection at the Instituto de Microbiología y Zoología Agrícola, Instituto Nacional de Tecnología Agropecuaria (INTA), Argentina. The fungi were preserved at −20°C in tubes containing media developed by Butler18 and at 4°C in glycerol media. Table 1 shows the strains of Cladorrhinum spp. included in this study.
Cladorrhinum spp. strains used in this study
| Species | Strain code | Isolation source |
|---|---|---|
| C. bulbillosum | INTA-AR 54 | Soybean crop; Buenos Aires province, Argentina (S 34° 36′ W 58° 40′) |
| C. bulbillosum | INTA-AR 104 | Fallow land; Buenos Aires province, Argentina (S 34° 36′ W 58° 40′) |
| C. bulbillosum | CBS 304.90 | Sand; Western Desert, Oasis Dakhla, Egypt; reference culture from holotypus |
| C. foecundissimum | CBS 180.66 | Soil; Netherlands; reference culture from neotypus |
| C. foecundissimum | MUCL 6980 | Triticum sativum soil; Schleswig-Holstein, Kiel, Kitzeberg, Germany |
| C. foecundissimum | CBS 341.92 | Maryland, Beltsville, USA |
| C. samala | INTA-AR 156 | Soybean crop; Santa Fe province, Argentina (S 31° 36′ W 60° 47′) |
| C. samala | INTA-AR 1 | Alfalfa crop; Buenos Aires province, Argentina (S 34° 36′ W 58° 40′) |
| C. samala | INTA-AR 20 | Alfalfa crop; Buenos Aires province, Argentina (S 34° 36′ W 58° 40′) |
| C. samala | CBS 302.90 | Triticum sativum soil; Western Desert, Oasis Dakhla, Egypt; reference culture from neotypus |
Carbon assimilation was investigated using Biolog FF MicroPlates. These plates are especially developed for cultivating filamentous fungi through the 95 individual carbon source utilization analysis (Biolog Inc. USA). The FF-IF broth (filamentous fungi-inoculation fluid) was prepared in a borosilicate test tube by mixing 0.25% Phytagel (P8169, Sigma) and 0.03% Tween 40 (P1504, Sigma) in distilled water. The solution was stirred until all the components were dissolved and sterilized by autoclaving for 20min at 121°C. Biolog FF MicroPlates (cat. no. 1006) were stored at 4°C until use. Pure cultures from the frozen stocks of Cladorrhinum spp. were firstly subcultured onto Potato Dextrose Agar (PDA) and then onto Malt Extract Agar (MEA) at 25°C. To promote sporulation, strains of Cladorrhinum spp. were incubated for 20 days under UV light with 12-hour photoperiod. Conidia were collected with sterile cotton-tipped swabs and suspended in a 16ml tube containing sterile IF-FF broth. The suspension was agitated in a vortex mixer for about 5 s and the inoculum density was adjusted to 75% transmittance at 590nm wavelength. Three Biolog FF MicroPlates, which contain 95 individual carbon sources, were inoculated with the conidial suspension of each isolate and incubated at 25°C in the dark. After 96h incubation, absorbance readings were taken at 750nm, which corresponds to turbidity reflecting mycelial production10. It was done in a microplate reader Emax™ (Molecular Devices®, Inc., Sunnyvale, CA, USA).
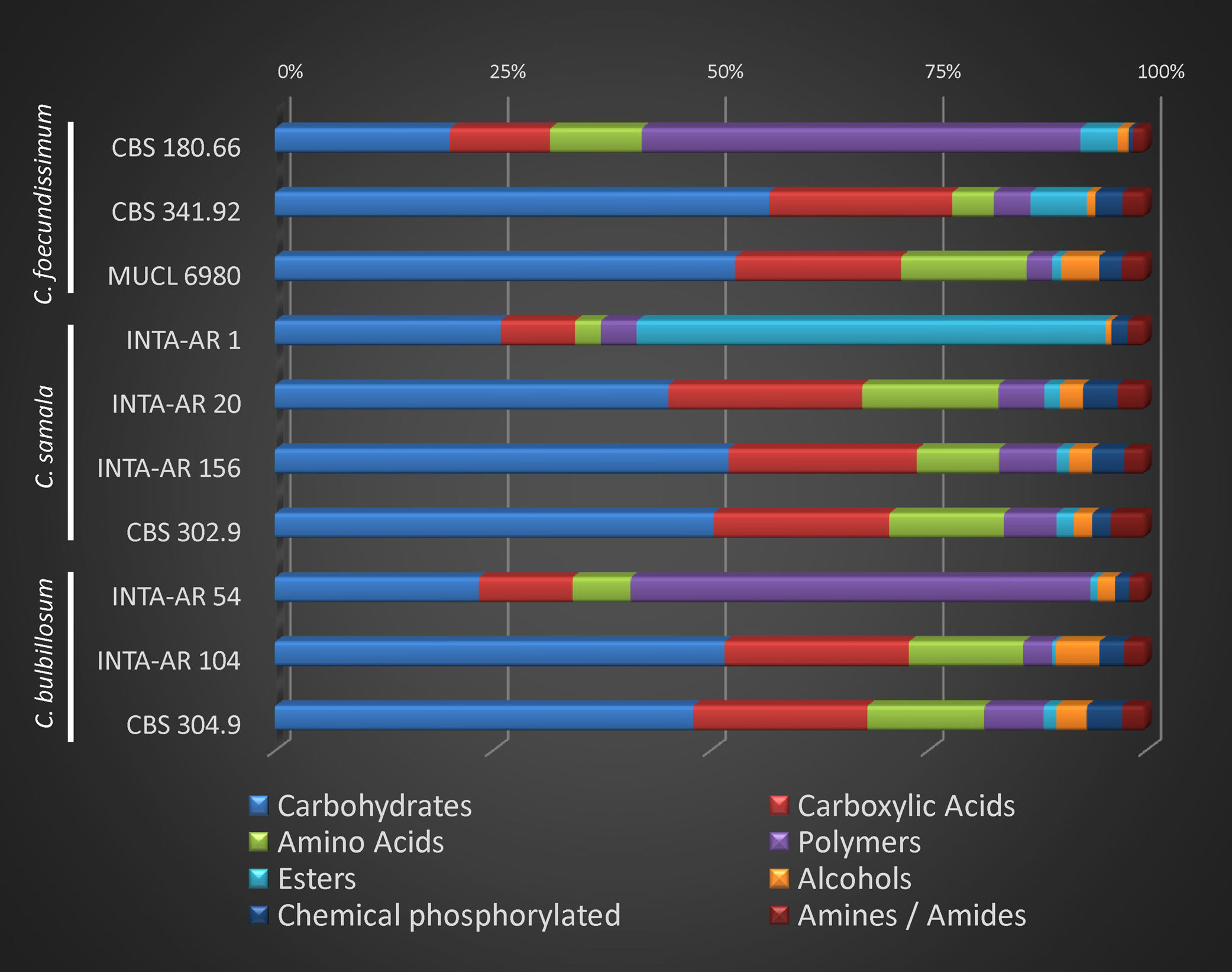
Statistical analyses were performed using InfoStat Software4. Absorbance values in each well of Biolog FF MicroPlates after 96h incubation were used instead of binary data to perform statistical analyses15. The optical density (OD) values of Biolog FF MicroPlates wells were corrected considering the background color developed in control well A1. Negative scores were set to zero. The average well color development (AWCD) was obtained as the sum of absorbance units of all positive wells divided by their total number. The average plate value was calculated using the media in triplicate. In order to reduce the variable-to-sample ratio in the microplates, the 95 carbon individual sources were grouped into eight chemical groups (carbohydrates, carboxylic acids, esters, polymers, alcohols, chemical phosphorylated, amines/amides, and amino acids). The average absorbance for the wells corresponding to each group was calculated2.
An analysis of variance of a factor and contrast (p < 0.05) using the least significant difference (LSD) was applied to demonstrate whether the AWCD of fungal strains was differential. Ten Cladorrhinum spp. strains were characterized using Biolog FF MicroPlates to obtain data on C-substrate utilization. The results obtained from the analysis of variance indicated F2.24: 14.48 (p < 0.0001).
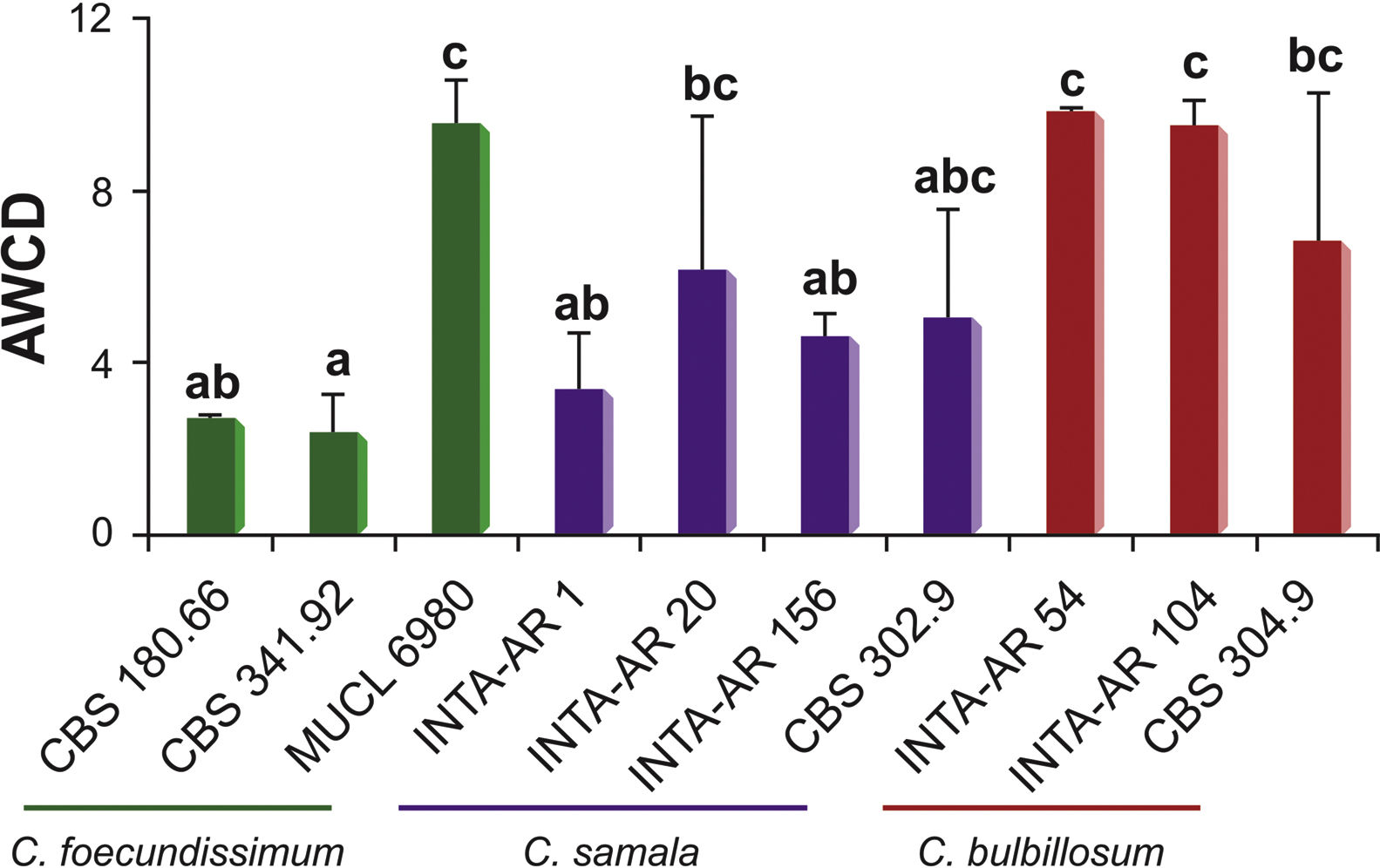
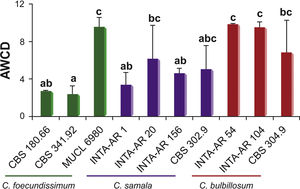
Figure 1 shows mycelial production (estimated by measuring average well color development [AWCD] at 750nm) by several Cladorrhinum spp. strains. While the strains C. foecundissimum MUCL 6980, C. samala INTA-AR 20, C. bulbillosum INTA-AR 54, INTA-AR 104 and CBS 304.90 revealed the highest biomass levels, the lowest biomass production was measured for isolate C. foecundissimum CBS 341.92. Based on the intraspecific responses, C. foecundissimum strains showed more variability than the C. bulbillosum and C. samala strains tested.
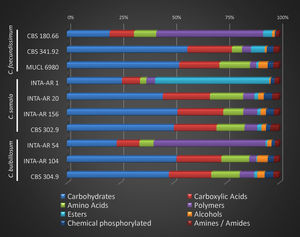
Relative consumption of several C compounds by Cladorrhinum spp. strains is reported in Figure 2. Carbohydrates were mainly consumed (over 45%) by most strains, with the exception of C. foecundissimum CBS 180.66, C. samala INTA-AR 1, and C. bulbillosum INTA-AR 54, which used mainly esters or polymers.
The comparison of the relative use of carbon sources by 10 strains belonging to three Cladorrhinum species using Biolog FF MicroPlates revealed variability among these abilities. Moreover, differences were found when several strains from the same species were compared. Although similar working strategies were reported as screening and evaluation tools for the physiological characterization of bacterial and fungal strains5, no data are available about the use of the microplates method for studying the biology of Cladorrhinum species. This Biolog FF MicroPlates analysis proved that all the strains tested might be considered different individuals due to specific biomass levels.
The results indicate that there was no species-specific behavior associated with the group of C-source assimilation in all the strains. Even though the preferential utilization of carbohydrates might be explained by the fact that carbohydrates and carboxylic acids are the primary sources for cellular metabolism8, other carbon sources such as amino acids also contributed to growth in the strains. The total consumption of these three compounds was 75% for most strains. The strains could be divided into three groups which were associated with: (a) intermediate to low mycelial production (C. samala INTA-AR 1 and INTA-AR 156, C. foecundissimum CBS 180.66 and CBS 341.92); (b) higher production of biomass, such as that found in C. bulbillosum (INTA-AR 54, INTA-AR 104, and CBS 304.90) and C. foecundissimum MUCL 6980; and (c) highly variable production, such as that found for some strains of C. samala (INTA-AR 20 and CBS 302.90). The C. bulbillosum INTA-AR 54 strain presented nutritional preferences for polymers, and C. samala INTA-AR 1 was differentiated by ester consumption in the group with low biomass production. In a taxonomic study analyzing the growth response by temperature, Madrid et al.11 reported a lower growth for the C. foecundissimum CBS 180.66 strain than for C. samala CBS 302.90 and C. bulbillosum CBS 304.90. Carmarán et al.3 observed the same trend for the strains analyzed in the present study. The existence of intraspecific variability in C. foecundissimum and C. samala is remarkable. It is known that microorganisms including fungi use certain C-substrates to increase biomass and for housekeeping reactions needed for fungal survival9. The differences found between the strains studied could be explained, in part, by the balance between the metabolism for growth and for fungal survival. Since several strains of C. foecundissimum and C. samala have potential as biocontrol agents against important fungal phytopathogens7, the ability of specific isolates to assimilate certain C sources might be related to their competitiveness under specific ranges of nutritional conditions. Variability in carbon source utilization may be associated with different ecological behaviors.
Likewise, the functions of organisms in an ecosystem are influenced by the environment, and the particular traits of these organisms are their nutrition mode, host or substrate preference, and specificity. Rice and Currah13 reported that the differences observed between strains within a species reflect ecological differentiation or adaptation to different habitats. This behaviour suggests that the colonization of roots in different crops by certain Cladorrhinum spp. isolates might be related to an adaptative specialization. According to Sagara14, C. foecundissimum is a representative component of the ecophysiological group “ammonia fungi”. The ability of these fungi to use amino acids as C-source could be indicative of their possible role in the ammonification processes at the rhizosphere level. The liberation of ammonium by Cladorrhinum spp. strains could be relevant since it could represent an additional role of these fungi in the promotion of plant growth. The use of different compounds containing low-molecular-mass nitrogen by these fungi, could play a role in the interaction of Cladorrhinum spp. strains and roots and their effect on the plant promoting growth.
In agreement with Kubicek et al.10, who worked with Trichoderma harzianum strains, our physiological data did not reflect the taxonomical delimitation of Cladorrhinum spp. species. A similar situation was observed when morphological and physiological features were used to separate strains of Trichoderma spp. selected for biological control activity against phytopathogens1,12.
To conclude, the physiological behavior of the studied Cladorrhinum spp. strains did not correspond to the taxonomic delimitation of the species. Further research is needed to correlate the high intraspecific variability found in the requirements of carbon sources related to the ecological behavior of the strains.
Conflict of interestThe authors declare that they have no conflicts of interest.
Financial supportINTA PROJECTS PNPV-1135023, PNAIyAV-1130034, PICT 2015-1620.
This work was funded by the Instituto Nacional de Tecnología Agropecuaria (INTA) through the following projects: PNPV-1135023 and PNAIyAV-1130034 and by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) of the Ministerio de Ciencia, Tecnología e Innovación Productiva through the project PICT 2015-1620 (M. C. N. Saparrat), CONICET (PUE INFIVE), CICPBA and UNLP, Argentina. Martin, M. is a recipient of a scholarship from CONICET, Argentina. Saparrat, M. C. N. is a researcher from CONICET, Argentina.