Bovine viral diarrhea virus (BVDV) is an important cause of economic losses worldwide. E2 is an immunodominant protein and a promising candidate to develop subunit vaccines. To improve its immunogenicity, a truncated E2 (tE2) was fused to a single chain antibody named APCH, which targets to antigen-presenting cells. APCH-tE2 and tE2 proteins were expressed in the baculovirus system and their immunogenicity was firstly compared in guinea pigs. APCH-tE2 vaccine was the best one to evoke a humoral response, and for this reason, it was selected for a cattle vaccination experiment. All the bovines immunized with 1.5μg of APCH-tE2 developed high levels of neutralizing antibodies against BVDV up to a year post-immunization, demonstrating its significant potential as a subunit vaccine. This novel vaccine is undergoing scale-up and was transferred to the private sector. Nowadays, it is being evaluated for registration as the first Argentinean subunit vaccine for cattle.
El virus de la diarrea viral bovina (BVDV) es causante de importantes pérdidas económicas a nivel mundial. La proteína E2 es la inmunodominante del virus y es la candidata para desarrollar vacunas de subunidad. Para mejorar su inmunogenicidad, una versión truncada de la E2 (tE2) se fusionó a un anticuerpo de cadena simple (APCH), que se dirige a las células presentadoras de antígeno. Se expresaron las proteínas APCH-tE2 y tE2 en el sistema de baculovirus y su inmunogenicidad fue evaluada y comparada en cobayos; la proteína APCH-tE2 fue la que indujo la mejor respuesta humoral. Por dicha razón se la evaluó en bovinos utilizando 1,5μg de antígeno. Los animales presentaron altos títulos de anticuerpos neutralizantes contra BVDV hasta un año posinmunización. Esta nueva vacuna está en proceso de escalado y se transfirió al sector privado. Actualmente se está evaluando para su registro como la primera vacuna argentina de subunidad para bovinos.
Bovine viral diarrhea virus (BVDV) is a common bovine pathogen that belongs to the family Flaviviridae, Pestivirus genus. Several clinical conditions, ranging from subclinical to severe disease, have been associated with this agent. Infections with BVDV are endemic in cattle populations worldwide and result in major economic losses. These losses are a result of high prevalence in combination with the negative effects on reproduction and the general health condition in affected herds.
Because most cattle are exposed to BVDV during their lifetime, vaccination programs are used extensively to protect against the consequences of infection2. However, there is concern that conventional vaccines (attenuated or inactivated formulations) may not be optimal for controlling BVDV infection15. For that reason, the possibility of using immunodominant proteins of BVDV in the form of subunit vaccines has gained widespread interest.
In Argentina, current viral vaccines containing BVDV are inactivated formulations combined with other viral and bacterial pathogens used for the prevention of infectious diseases belonging to the reproductive and respiratory complex. In this respect, these vaccines present another limitation at the industry scale related to obtaining enough BVDV viral antigen. Moreover, while it is believed that inactivated vaccines are safe, the emergence of a new fatal disease named neonatal pancytopenia, associated with the use of inactivated vaccines against BVDV, has been reported in several European countries. Marbin Darbin Bovine Kidney (MDBK) cells are commonly used to produce BVDV stocks, which are sometimes concentrated during the manufacturing process of vaccines. Pancytopenia is triggered when vaccinated animals develop autoantibodies due to the large amount of MDBK cellular debris present in the formulations1. To face all these difficulties, it is undisputable that there is a need for developing new generation of efficient and safe vaccines.
Subunit vaccines are safe and could allow discrimination between infected and vaccinated animals (DIVA). The disadvantage of using such vaccines is the difficulty in engineering them to generate an adequate protective immune response at a cost that is practical for veterinary applications. In that regard, considerable effort is under way to devise methods of enhancing the immunogenicity of such vaccines. One of the most successfully employed strategies to date is based on targeting the encoded antigens to the sites of immune induction, by using single-chain variable fragments (scFv) of antibodies that specifically recognize an invariant epitope of the major histocompatibility complex class II DR molecule on the surface of antigen-presenting cells. This strategy has demonstrated to be very efficient in improving the immune responses induced against many different antigens, either using recombinant subunit proteins or DNA vaccination5,13.
E2 is the major glycoprotein of BVDV envelope and the most immunogenic protein of this virus. Neutralizing antibodies induced in infected animals are mainly directed against E26. Thus, the use of immunodominant BVDV protein (E2) as a subunit vaccine may be a useful tool for the development of efficient strategies to control the virus10,14. The baculovirus-insect cell expression system (Bevs, the abbreviature of Baculovirus expression vector system) is one of the most widely used tools for the high level expression of heterologous proteins in a eukaryotic host and was chosen to express tE2 and APCH-tE2 immunogens in this work. Advantages include the production of high level foreign proteins as well as the insect cells capable of post-translational modifications similar to those of mammalian cells. In addition, the Bevs system is safe, easy to use and readily amenable to scale-up4. Up to now, several vaccines produced in insect cells are commercially available for veterinary and human use, which were developed against cervical cancer, prostate cancer, classical swine fever virus and porcine circovirus3.
To assess the enhancing effect of APCH on E2 glycoprotein immunogenicity, expression cassettes encoding either a truncated form of E2 (tE2) or tE2 fused to the scfvAPCH (APCH-tE2) were constructed using the pFast Bac Dual vector (Invitrogen, USA). The signal peptide melittin was added to both constructions in order to secrete the recombinant proteins into the insect cell culture supernatants. The constructs were sequenced to confirm their integrity prior to generating recombinant baculoviruses (Macrogen, Inc., Korea), which were generated by the Bac-to-Bac System (Invitrogen, USA).
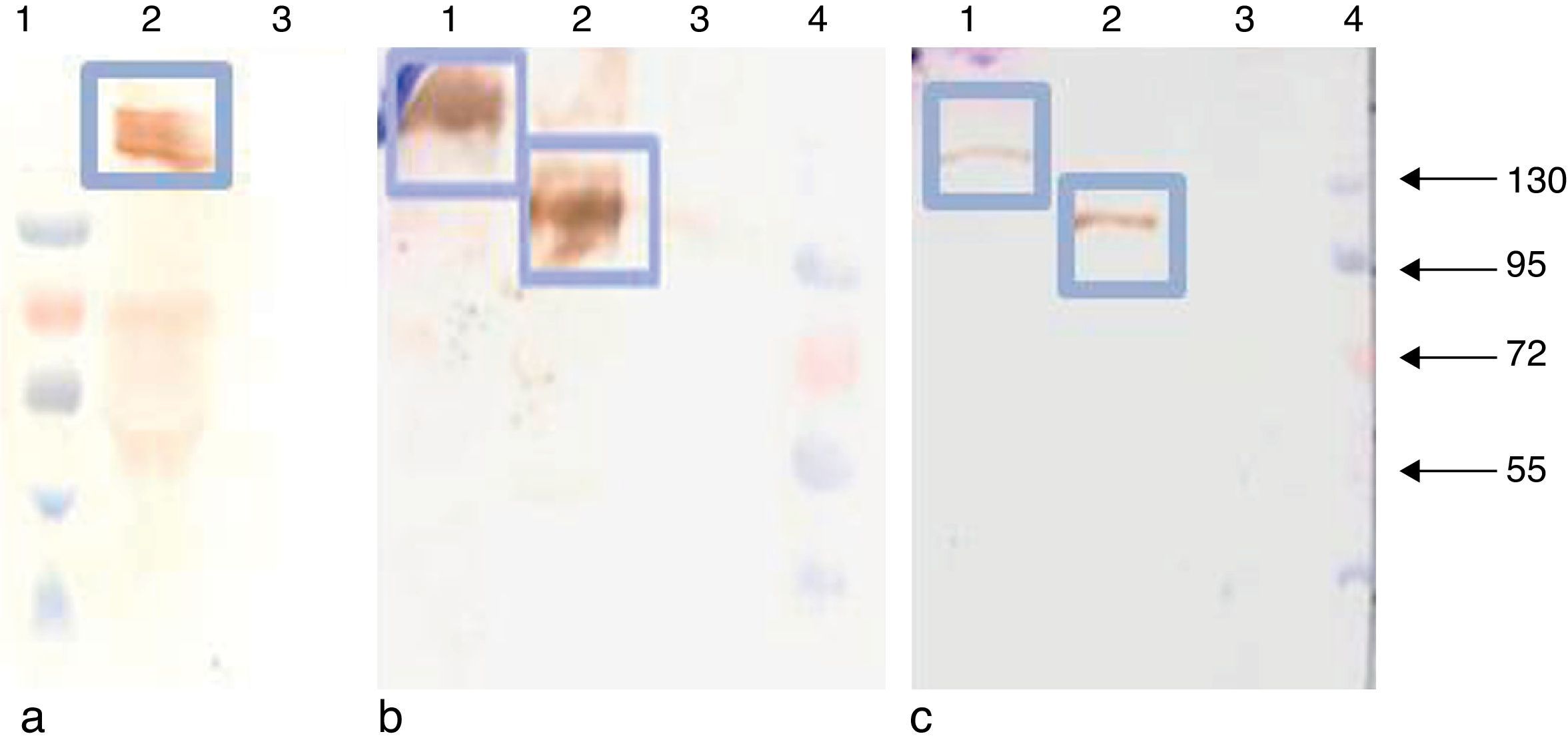
APCH-tE2 and tE2 were successfully expressed and secreted into the supernatants of Spodoptera frugiperda (SF9) and High Five™ (H5) insect cells (Invitrogen, USA). Both cell lines were cultured in monolayers and spinner flasks and showed similar expression levels, reaching 5mg/l and 12mg/l for APCH-tE2 and tE2 respectively, as assessed by ELISA9. Moreover, it could be verified by Western blot that the recombinant proteins were specifically recognized by a monoclonal antibody against E2 protein (Fig. 1).
Detection of recombinant proteins tE2 and APCH-tE2 by Western blot analysis under non-reducing conditions in 10% polyacrylamide gels. (a) Monoclonal antibody (mAb) anti E2 2.9H. Lane 1: prestained molecular weight (PageRuler™, Fermentas), lane 2: dimer of APCHt-E2 (150kDa), lane 3: negative SF9 supernatant. (b) mAb anti E2 CA1. Lanes 1 and 2: dimers of APCH-tE2 and tE2 (150 and 100kDa), lane 3: negative SF9 supernatant, lane 4: molecular weight marker. (c) mAb anti-histidine (Penta His, Qiagen). Lanes 1 and 2: dimers of APCH-tE2 and tE2, lane 3: negative SF9 supernatant, lane 4: molecular weight marker. The arrows show the molecular weight of each stained band with their values expressed in kDa.
The guinea pig model is currently used to test killed commercial formulations of reproductive and respiratory complex vaccines in Argentina (SENASA Resolution No. 589/12 related to animal health); since the levels of neutralizing antibodies induced in guinea pigs were statistically validated as a reliable indicator to predict vaccine immunogenicity in bovines7. For that reason, this animal model was chosen to test the vaccines developed in this work.
Briefly, 35 male guinea pigs strain SSI:AL, 12 weeks old, were obtained from the animal care facilities of the National Institute of Agricultural Technology (INTA). The animals were randomly divided into six groups (5 animals per group) prior to experimentation and immunized with SF9 supernatants containing: (i) 1μg of tE2, (ii) 0.5μg of tE2, (iii) 0.2μg of tE2, (iv) 1μg of APCH-tE2, (v) 0.5μg of APCH-tE2 and (vi) 0.2μg of APCH-tE2. A negative control group (n=5) was inoculated with supernatants from non-infected SF9 cells. Vaccines were formulated with oily adjuvant (w/o Montanide ISA 50, Seppic, France) containing the antigens in a proportion adjuvant:antigen of 60:40. The vaccines were applied intramuscularly (i.m.) in a volume of 0.6ml on days 0 and 21. Blood samples were collected on days 0, 30 and 60 post immunization (dpi) and sera were evaluated by virus neutralization (VN), following the protocol described elsewhere8. Neutralizing antibody titers were determined against the Singer strain by the method of Reed and Muench11.
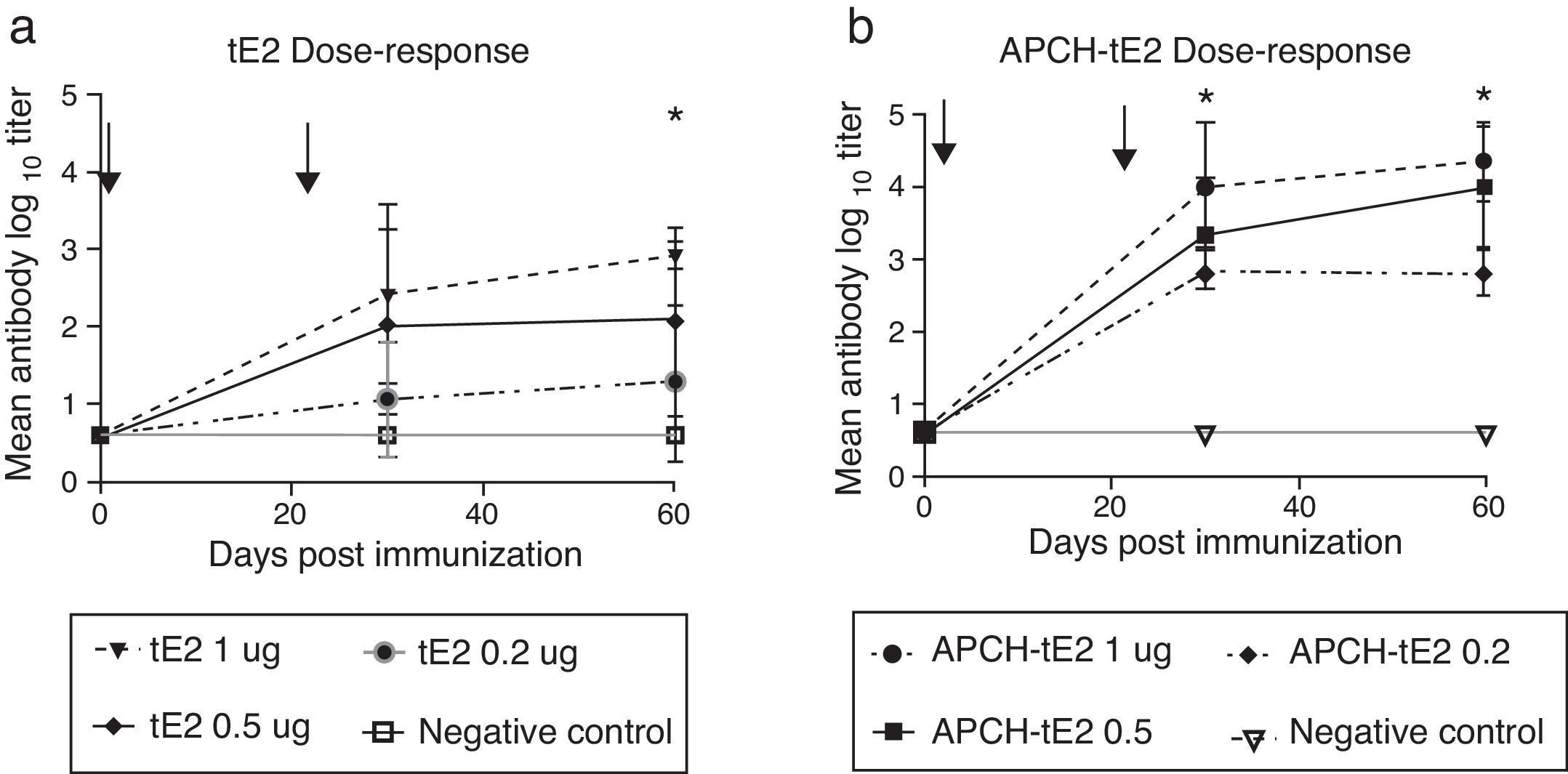
The vaccine based on APCH-tE2 was significantly more efficient in the induction of Nabs than the one based on tE2 protein alone. This could be better appreciated in the lower doses evaluated (0.2μg and 0.5μg) (Fig. 2). For this reason, the vaccine based on the fusion protein was chosen to perform the cattle vaccination experiment.
Dose–response of tE2 and APCH tE2 in guinea pigs. Experimental groups of guinea pigs (n=5) were immunized with oily vaccines containing 0.2, 0.5 or 1μg of tE2 (a) or APCH-tE2 (b). A negative control group was immunized with a negative SF9 supernatant. Animals were bled on 30 and 60 days post immunization (dpi) and sera were evaluated by VN. Arrows indicate times of vaccination. The results of the VN were statistically evaluated by analysis of variance (ANOVA), determining significant differences between groups. Statistical significance was assessed at p <0.05 for all comparisons, using the Statistix 8.0 Analytical Software.
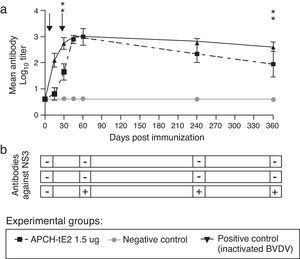
To that end, 18 8–12 month old Aberdeen Angus calves weighing 160–200kg were obtained from INTA's closed herd. All animals were checked for the absence of BVDV-specific Nabs by VN and BVDV RNA by RT-PCR. Bovines were randomly divided into 3 groups and immunized i.m. with 3ml of the oily vaccine containing: (i) 1.5μg of APCH-tE2, (ii) inactivated BVDV Singer strain 1×106.5TDIC/ml or (iii) a BVDV non-related protein; on days 0 and 30. Sera from all animals were sampled on 15, 30, 45, 60, 240 and 360dpi and evaluated by VN. Furthermore, a commercially available competitive NS3 antibody ELISA kit (Ingenasa, Spain) was used and serum samples from the three experimental groups of bovines were processed following the manufacturer's instructions at 0, 60, 240 and 360dpi.
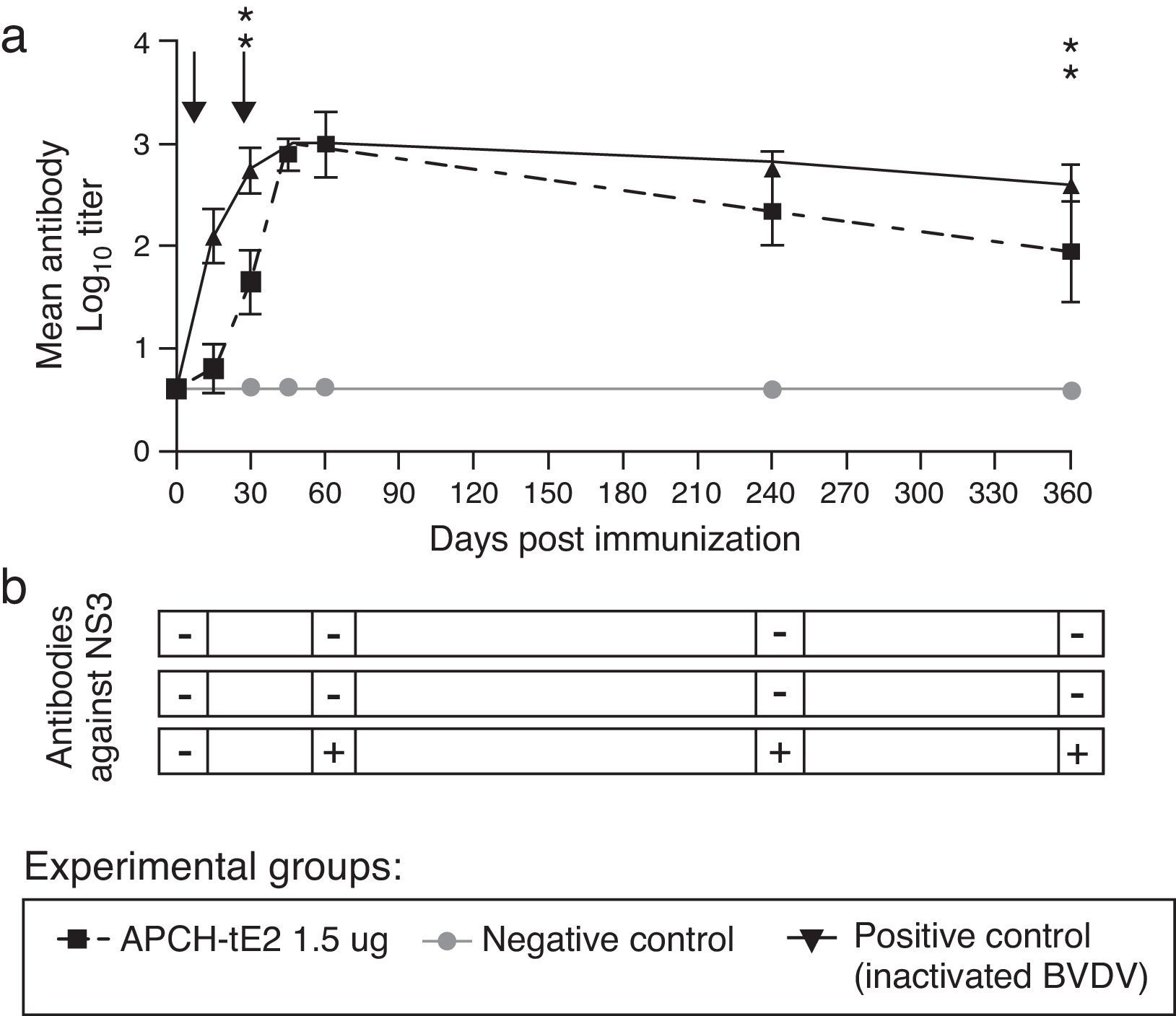
Results of VN showed that bovines vaccinated with either 1.5μg of APCH-tE2 or an inactivated BVDV showed geometric mean titers (GMT) >2, which were maintained at least for one year (Fig. 3a). Negative control animals did not develop Nabs against BVDV in all the time points analyzed; therefore, BVDV circulation throughout the experiment was discarded. Furthermore, only the animals from the positive control group showed anti NS3 antibodies, highlighting the ability of the vaccine to be used as a DIVA tool (Fig. 3b). None of the vaccinated bovines showed local reactions or adverse effects throughout the trial.
Vaccination experiment in cattle. (a) Calves were immunized twice with 1.5μg of APCH-tE2. On 15, 30, 45, 60, 240 and 360 days post immunization (dpi) animals were bled and sera were evaluated by VN. A positive control group was immunized with an inactivated vaccine containing 106.5TDIC50/ml BVDV, Singer strain and a negative control group was vaccinated with a formulation of a negative SF9 supernatant. Arrows indicate times of vaccination. Differences in the geometric mean titer (GMT) for neutralizing antibodies among groups were evaluated by ANOVA under a model of repeated measures throughout time, followed by a general contrast post ANOVA test. Statistical significance was assessed at p <0.05 for all comparisons. (b) Antibody response against BVDV NS3 (p80) was determined using the commercial NS3-ELISA Ingenzim BVD Compac kit, (Ingenasa, Spain); +: positive, −: negative. Cattle management, inoculation, and sample collection were conducted by trained personnel under the supervision of a veterinarian and in accordance with protocols approved by INTA's Ethical Committee of Animal Welfare (CICUAE, Protocol 23/2012).
Mechanisms underlying protection against BVDV infection are not completely understood; however, the presence of Nabs and the effective priming of humoral memory appeared to be important factors in preventing and controlling BVDV infection12. In this work, APCH-tE2 emulsified in an oily adjuvant induced a strong humoral response in cattle (Fig. 3), which was comparable to those required for a satisfactory BVDV killed vaccine according to CFR (Code of Federal Regulations) 113.215. The low dose of immunogen administered and the long-lasting humoral immunity elicited by bovines vaccinated with APCH-tE2 (at least up to one year post immunization) demonstrate that subunit recombinant vaccines can be optimized in order to avoid high protein doses or repeated boosters to induce an acceptable immune response. Such vaccines, which are widely applied in public health as well as in pets, pig farming and aviculture, have not yet been extended to cattle, not having to date recombinant vaccines on the market. Furthermore, lack of anti NS3 antibodies in animals vaccinated with APCH-tE2 reliably indicates that the vaccine can be accurately used as a DIVA tool, since both natural BVDV infection and the use of some killed or attenuated vaccines elicit the production of antibodies against that protein, among others2.
Given these encouraging results, this novel vaccine is currently undergoing scale-up and was transferred to the private sector. Nowadays, it is being evaluated for registration as the first Argentinean subunit vaccine for cattle.
Moreover, we are currently working to enhance this subunit vaccine by adding the recombinant E2 glycoprotein from other BVDV genotypes in order to achieve broader BVDV antigenic coverage. Future studies will be focused on performing a vaccination-challenge assay with this novel formulation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by Ethical Committee of Animal Welfare (CICUAE).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors are grateful to Dr. Osvaldo Zabal and his group for providing valuable help on cell culture and to Dr. Oscar Taboga and Dr. Gabriela Lopez for assistance in protein expression. Financial support was received through INTA's Specific ProjectAEGR 2413.