Adult chinchillas (Chinchilla lanigera) that had suddenly died in a commercial farm located in La Plata City, Buenos Aires Province, Argentina, in July 2012 were macroscopically, histopathologically, and microbiologically examined. Salmonella enterica serovar Typhimurium (S. Typhimurium) was isolated from the liver, spleen, heart, lungs, kidneys and intestines from each of the five animals evaluated. The five strains were susceptible to ampicillin, cephalotin, cefotaxime, nalidixic acid, gentamicin, streptomycin, chloramphenicol, fosfomycin, nitrofurantoin and trimethoprim-sulfamethoxazole, and resistant to tetracycline. Each of the five S. Typhimurium isolates was analyzed by XbaI- pulsed-field gel electrophoresis (PFGE), showing an identical electrophoretic profile with 15 defined bands, which was found to be identical to pattern ARJPXX01.0220 of the PulseNet Argentine National database of Salmonella PFGE patterns. This is the first work describing the postmortem diagnosis of an outbreak of salmonellosis in chinchillas by using molecular methods such as PFGE.
Empleando estudios anatomopatológicos y microbiológicos se examinó a un grupo de chinchillas (Chinchilla lanigera) adultas que murieron súbitamente en 2012 en una granja de la ciudad de La Plata (Buenos Aires, Argentina). Se aisló Salmonella enterica serovar Typhimurium (S. Typhimurium) del hígado, el bazo, el corazón, los pulmones, los riñones y los intestinos de los cinco animales evaluados. Los cinco aislamientos estudiados (uno por animal) fueron sensibles a ampicilina, cefalotina, cefotaxima, ácido nalidíxico, gentamicina, estreptomicina, cloranfenicol, fosfomicina, nitrofurantoína y trimetoprima-sulfametoxazol, y resistentes a tetraciclina. El análisis de dichos aislamientos por electroforesis en gel de campo pulsado [pulsed-field gel electrophoresis (PFGE)] con XbaI mostró un perfil electroforético idéntico con 15 bandas, idéntico a su vez al patrón ARJPXX01.0220 del banco nacional argentino de datos de PulseNet, que cuenta con patrones de PFGE de Salmonella. El presente trabajo describe por primera vez el diagnóstico postmortem de un brote de salmonelosis en chinchillas usando un método molecular, como la electroforesis en gel en campo pulsado.
Salmonellosis is a widespread zoonotic and foodborne disease that represents one of the most important global veterinary and public health threats. Salmonella enterica serovar Typhimurium (S. Typhimurium) represents one of the main causes of salmonellosis in both humans and animals worldwide11. Chinchillas (Chinchilla lanigera) are hystricomorphic mammals which belong to the order Rodentia and family Chinchillidae and are native of the South American Andes Mountains, having natural and restrictive distribution in Peru, Bolivia, and Chile6. The species C. lanigera was domesticated and now there are millions of chinchillas in captivity throughout the world. Chinchillas are raised and exhibited in zoos, used as exotic pets, and bred in farms for the commercial use of their pelts6,10.
Salmonella spp. isolates can be characterized by phenotyping methods and by genotyping methods such as pulsed-field gel electrophoresis5,11 (PFGE). The PFGE method uses rare cutting restriction enzymes that generate a large number of DNA fragments, whose patterns are highly specific for molecular subtyping when separated by agarose gel electrophoresis5,13. Thus, PFGE, which has not been previously applied to confirm the diagnosis of salmonellosis in chinchillas or to clarify epidemiologic and phylogenetic aspects of Salmonella strains associated with outbreaks or individual cases in this animal species, represents one of the best genotyping and subtyping methods for members of the genus Salmonella5,11,13.
The objective of our study was to characterize the S. Typhimurium strains associated to an outbreak that occurred on a chinchilla farm.
In the last decades, S. Typhimurium and Salmonella enterica serovar Enteritidis (S. Enteritidis) have emerged as the main causes of human foodborne illnesses worldwide and have been recognized as the most prevalent causes of Salmonella infections in humans in the USA and Europe1,4. According to the information reported by the Argentine National Reference Laboratory and the WHO Global Foodborne Infections Network, the same situation was observed in Argentina, where S. Typhimurium has been the most common serovar isolated from human infections since 2007. Moreover, Argentina is known as one of the leading producers of chinchilla pelts worldwide and salmonellosis is one of the most important infectious diseases affecting chinchilla production2.
Salmonellosis has been described in chinchillas since 196614. The first report of salmonellosis in chinchillas mentioned an outbreak of Salmonella enterica serovar Dublin (S. Dublin) on a chinchilla farm in the United Kingdom14. This British study described that the affected chinchillas showed loss of body condition, enteritis, diarrhea, and sudden death. S. Dublin was isolated from three out of the nine chinchillas evaluated. Due to the fact that the strains showed antibiotic susceptibility to chloramphenicol, furazolidone, neomycin, ampicillin, and framycetin, antibiotic treatment with chloramphenicol was implemented and the outbreak was successfully controlled. The authors of this study concluded that the S. Dublin outbreak in British farmed chinchillas could have been associated with recently introduced carrier breeders and highlighted the possible infective role of one of the farmers because of its S. Dublin confirmed carrier status14. Another British study carried out in 1989 briefly summarized the isolation of Salmonella enterica subsp. arizonae recovered from a pet chinchilla with septicemia and identified as the rare serotype O-21 r, z8. In Slovakia, a surveillance of bacterial diseases in several commercial large-scale chinchilla farms was reported in 1994, and both S. Enteritidis and Listeria spp. infections were found to be the main causes of death in farmed chinchillas10. In Japan, a S. Enteritidis strain was isolated from most of the organs and intestinal content of a pet chinchilla affected by septicemia in 199715. The postmortem and microscopic evaluations of this pet chinchilla revealed meningitis, panophthalmitis, necrotic enteritis, focal hepatic necrotic lesions and microvascular thrombosis in the spleen and kidneys, which were associated with the bacterial infection since S. Enteritidis colonies were immunohistochemically detected in the affected pet chinchilla15. In Argentina, outbreaks of S. Typhimurium were reported on two chinchilla farms located in Jujuy Province and Santa Fe Province showing 12-30% of mortality in 19992. This Argentine study described farmed chinchillas suffering from anorexia, apathy, hyperthermia, diarrhea, abortion, and death. The postmortem examination of the affected animals revealed septicemic lesions in the liver, lungs, kidneys, stomach, intestines and uterus. The microbiological study allowed to isolate S. Typhimurium strains which were susceptible to a wide range of antibiotics in both cases. This study highlighted the control of both outbreaks with antibiotics such as furazolidone, chloramphenicol, and enrofloxacin and autogenous bacterins2. In Turkey, an outbreak caused by both S. Typhimurium and S. Enteritidis infections on a chinchilla farm was reported in 20027. This study described farmed chinchillas suffering from apathy, anorexia, tremors, paralysis, diarrhea, vaginal secretions, abortion, and death. The postmortem evaluation of two affected chinchillas revealed enteritis, hemorrhagic and purulent endometritis and septicemic lesions. This Turkish study reported the isolation of Salmonella strains which were susceptible to enrofloxacin, amoxicillin, and gentamicin, and resistant to erythromycin and ampicillin7. In Croatia, an outbreak of S. Enteritidis and the isolation of S. Sofia in farmed chinchillas was reported in 20039. The postmortem examinations made in this Croatian study revealed gastritis, enteritis, enlarged spleens with or without focal necrosis, livers with focal necrosis and endometritis in chinchillas from five different commercial farms. The authors of this study reported the isolation of S. Enteritidis from the five commercial farms evaluated, and S. Sofia from one of them. All S. Enteritidis isolates were susceptible to ampicillin, chloramphenicol, neomycin, enrofloxacin, and trimethoprim-sulfamethoxazole, and resistant to sulfamethoxazole, whereas only three S. Enteritidis isolates were resistant to tetracycline. This study highlighted the successful results of the antibiotic treatments with enrofloxacin and discussed possible routes of Salmonella infection for both humans and animals, considering that most of the animal-derived food groups and human-animal working contacts could represent serious threats for public and veterinary health9. In Brazil, a retrospective study of postmortem examinations of chinchillas (1997-2011) including 202 necropsied animals was reported in 2012. This study briefly described one animal affected by a Salmonella spp. infection associated with hepatitis and cholecystitis6.
Eight previous works have described salmonellosis in both pet and farmed chinchillas2,6–10,14,15, but none of them reported the application of molecular methods such as PFGE. Reports of outbreaks in farmed chinchillas or individual infections in pet chinchillas due to S. Typhimurium2,7, S. Enteritidis7,9,10,15, S. Dublin14, S. Sofia9, S. enterica subsp. Arizonae8, and Salmonella spp.6 allow to consider the high relevance of salmonellosis in chinchillas as a veterinary and public health threat worldwide, and the need for molecular methods such as PFGE to support its diagnosis and further investigations.
In our case, adult chinchillas suffered sudden death on a commercial farm located in La Plata City, Buenos Aires Province, Argentina, in July 2012. The outbreak lasted one week, during which 35% of the 3600 farmed chinchillas died. Each animal was housed in a stainless steel cage of standard size. The farmers provided running water using a hose system with one nipple per cage, and commercial chinchilla pelleted food which was stored in a peridomestic rodent-free covered container. Poor hygienic conditions were noted because abundant peridomestic rodent feces were observed close to several cages.
Five of the 1260 dead chinchillas were submitted to the Laboratorio de Diagnóstico de Enfermedades de las Aves y los Pilíferos (Cátedra de Patología de Aves y Pilíferos, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata) for their postmortem examination and diagnostic workup.
The postmortem examination revealed emaciated animals with hemorrhagic inflammation and presence of mucous and white nodules of approximately 2-3mm in diameter in the duodenum and stomach. The livers showed pale, dark, and gray color changes. Splenomegaly and hemorrhagic pneumonia were also observed in all the animals examined. Samples of the intestine, stomach, lungs, liver, kidneys, heart, and spleen from the five animals were taken at necropsy, fixed in 10% buffered formalin for 48h, embedded in paraffin, sectioned at 3μm, and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). Smears of tissues were stained using Gram and modified Ziehl-Neelsen (Z-N) methods.
Tissue samples were also inoculated onto Hektoen enteric agar plates (Becton Dickinson France S.A., Le Pont-de-Claix, France), Mac Conkey agar plates (Becton Dickinson France S.A.), and trypticase soy agar plates (Becton Dickinson France S.A.) with 5% sheep blood and then incubated at 37°C for 48h under aerobic conditions. In addition, these samples were inoculated onto Sabouraud agar plates (Merck KGaA, Darmstadt, Germany) and incubated at 28°C for 1 week under aerobic conditions. Presumptive Salmonella spp. colonies were chosen and biochemically tested. Species, subspecies and serotype were identified according to the White-Kauffmann-LeMinor scheme.
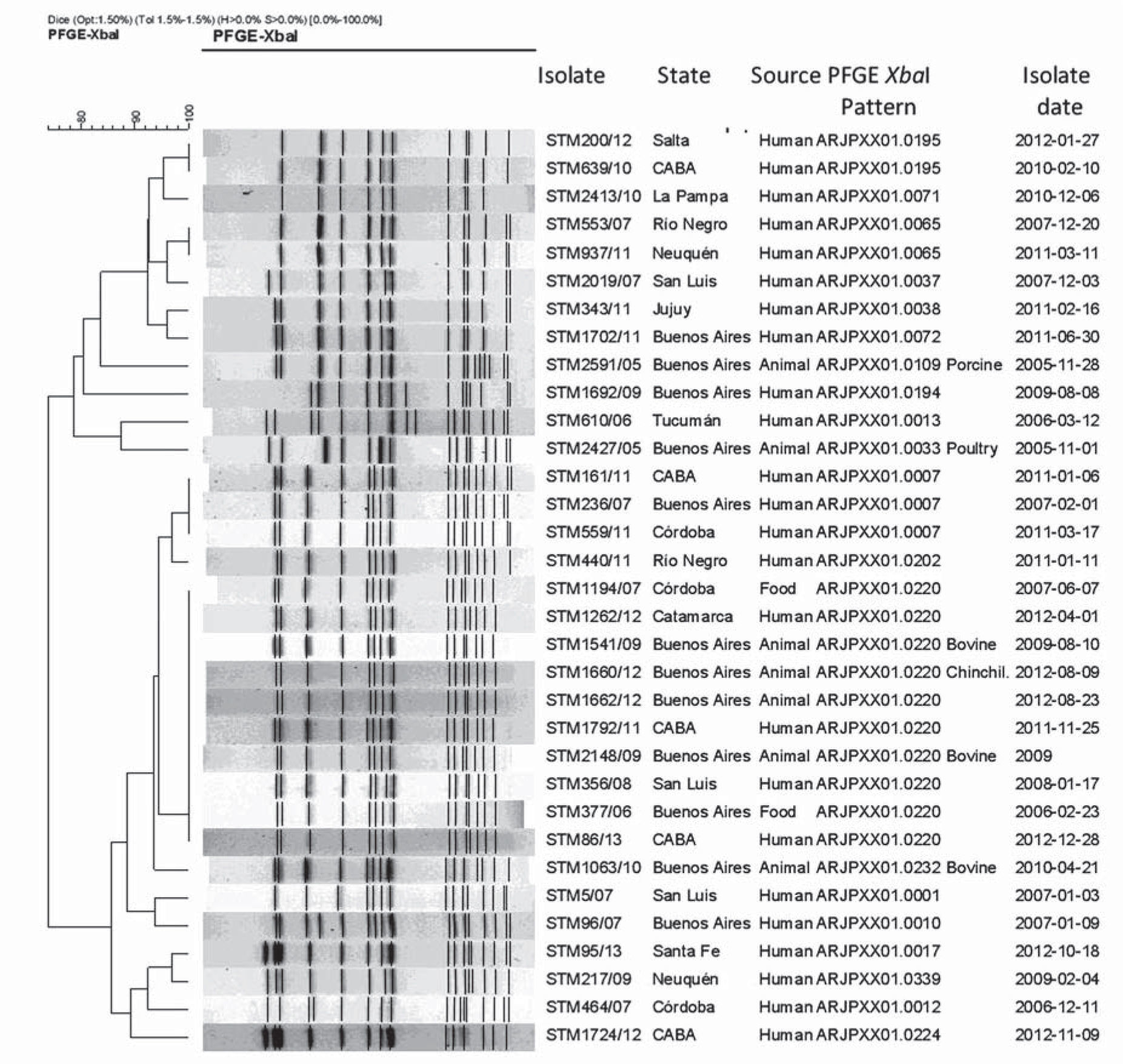
Susceptibility tests of Salmonella spp. isolates to ampicillin (10μg/disk), cefotaxime (30μg/disk), nalidixic acid (30μg/disk), gentamicin (10μg/disk), streptomycin (10μg/disk), chloramphenicol (30μg/disk), cephalotin (30μg/disk), nitrofurantoin (300μg/disk) and trimethoprim-sulfamethoxazole (1.25/23.75μg/disk) were performed following standardized disk diffusion methods for Enterobacteriaceae3. The relatedness among the isolates obtained from each of the five farmed chinchillas was determined by PFGE by applying the XbaI enzyme (New England Biolabs, Inc., Ipswich, MA), following the PulseNet standard protocol12 in a CHEF-DR III Pulsed Field Electrophoresis System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Gel images were obtained with a Gel-Doc 2000 Gel Documentation System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PFGE patterns were analyzed using BioNumerics software version 4.5 (Applied Maths, Kortrijk, Belgium). The relationship among the PFGE patterns was estimated using the same software, applying the Dice coefficient and the UPGMA method with 1.5% tolerance and 1.5% optimization parameters as recommended by PulseNet usual analysis. PFGE profiles of the S. Typhimurium isolates obtained from each of the five chinchillas in the present case were compared with the PulseNet Argentine National database of Salmonella PFGE patterns, which contains 364 unique XbaI-PFGE patterns corresponding to 1291 isolates of S. Typhimurium from different sources (human, animals, and the environment), obtained in Argentina from 1968 to 2013.
The H&E microscopic examination of lung tissues showed edema and severe infiltration of mononuclear inflammatory cells (macrophages, lymphocytes, and plasma cells) with diffuse distribution in alveoli and blood vessel congestion. The livers showed sinusoidal and great blood vessel congestion, small foci of necrosis, and small focal areas of mononuclear inflammatory cell infiltration with diffuse distribution. The spleens showed moderate lymphoid depletion. The stomachs had evident edema in the lamina propria, and severe mononuclear inflammatory cell infiltration in the mucous membrane, with epithelium hyperplasia. The intestines showed edema in the lamina propria and mononuclear inflammatory cell infiltration in the mucous membrane, with abundant bacterial colonies. The PAS and modified Z-N stains yielded negative results and no fungal growth was observed in Sabouraud agar plates.
The isolates obtained from each of the five farmed chinchillas were identified as S. Typhimurium. The isolates were susceptible to all the antimicrobial agents tested except to tetracycline. The five S. Typhimurium isolates were identical by XbaI-PFGE, showing an electrophoretic profile with 15 defined bands between 670 and 20 kilobases. This profile was found to be identical to pattern ARJPXX01.0220, representing 1.5% of the PulseNet Argentine National database of Salmonella PFGE patterns, which includes isolates from human, animal, and food sources. The XbaI-PFGE pattern was also related to a foodborne outbreak in a hospital in Buenos Aires Province in 2013 (Fig. 1).
In our case, the isolation of S. Typhimurium from the liver, spleen, heart, lungs, kidneys and intestines from each of the five adult farmed chinchillas indicated septicemic infections. The macroscopic and microscopic lesions observed in our study were associated with acute salmonellosis, as previously reported in this animal species2,7,9,15.
All isolates obtained in our study were resistant to tetracycline, which could be the result of overusing this antimicrobial agent against other bacterial infections. The S. Typhimurium strains isolated in our study showed low levels of antibiotic resistance, in accordance with the low levels of antibiotic resistance previously observed in Argentine and Turkish S. Typhimurium strains isolated from farmed chinchillas in 1999 and 2002, respectively2,7, and in contrast to the high levels of antibiotic resistance observed in S. Typhimurium strains isolated from humans, other animal species and foodstuffs previously reported worldwide1.
This is the first report confirming an outbreak of salmonellosis in chinchillas by molecular methods such as PFGE. The same clone of S. Typhimurium was isolated in the five farmed chinchillas evaluated in our study. Therefore, our results support the hypothesis that the animals acquired the infection from the same infectious source. Unfortunately, such source could not be established until now, but the presence of abundant feces of peridomestic rodents inside the farm could have played a key role in the transmission of the bacterial pathogen during this outbreak. Other possible routes of transmission could have been associated with the consumption of contaminated food or water, contact with human or animal carriers or combinations of these factors. The XbaI-PFGE pattern of the S. Typhimurium strains isolated from each of the five farmed chinchillas evaluated in our case has been identified since 2006 in samples from humans, domestic animals, and food from different regions of Argentina. This means that this clone is widely disseminated in Argentina. The PFGE pattern identified in the farmed chinchillas involved in this outbreak was immediately incorporated into the PulseNet Argentine National database of Salmonella PFGE patterns.
Due to the fact that people handling chinchillas or cleaning chinchilla accessories (e.g. cages) are likely to acquire Salmonella spp. infections, they should follow the recommended precautionary measures to reduce the transmission risk of this zoonotic microorganism.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
AuthorshipC. D. Gornatti Churria and G. B. Vigo contributed equally to this article and are joint first authors.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to dedicate this article to Germán B. Vigo who passed away during its writing.