Resistance to β-lactam/β-lactamase inhibitors in enterobacteria is a growing problem that has not been intensively studied in Argentina.
In the present work, 54/843 enterobacteria collected in a teaching hospital of Buenos Aires city were ampicillin-sulbactam-resistant isolates remaining susceptible to second- and third-generation cephalosporins. The enzymatic mechanisms present in the isolates, which were also amoxicillin-clavulanic acid (AMC)-resistant (18/54) were herein analyzed.
Sequencing revealed two different variants of blaTEM-1, being blaTEM-1b the most frequently detected allelle (10 Escherichia coli, 3 Klebsiella pneumoniae, 2 Proteus mirabilis and 1 Raoultella terrigena) followed by blaTEM-1a (1 K. pneumoniae). Amoxicillin-clavulanate resistance seems to be mainly associated with TEM-1 overproduction (mostly in E. coli) or co-expressed with OXA-2-like and/or SHV β-lactamases (K. pneumoniae and P. mirabilis).
A new blaTEM variant (TEM-163) was described in an E. coli strain having an AMC MIC value of 16/8μg/ml. TEM-163 contains Arg275Gln and His289Leu amino acid substitutions. On the basis of the high specific activity and low IC50 for clavulanic acid observed, the resistance pattern seems to be due to overproduction of the new variant of broad spectrum β-lactamase rather than to an inhibitor-resistant TEM (IRT)-like behavior.
La resistencia a la combinación de β-lactámico/inhibidor de β-lactamasa en enterobacterias es un problema creciente que no ha sido estudiado intensamente en Argentina.
En el presente trabajo, 54/843 enterobacterias recolectadas en un hospital universitario de la ciudad de Buenos Aires fueron resistentes a ampicilina-sulbactama, pero se mantuvieron sensibles a las cefalosporinas de segunda y tercera generación. Se analizaron los mecanismos enzimáticos presentes en los aislamientos que también fueron resistentes a amoxicilina-ácido clavulánico (AMC) (18/54).
La secuenciación reveló dos variantes diferentes de blaTEM-1, donde blaTEM-1b es el alelo más frecuentemente detectado (10 Escherichia coli, 3 Klebsiella pneumoniae, 2 Proteus mirabilis y 1 Raoultella terrigena), seguidos por blaTEM-1a (1 K. pneumoniae). La resistencia a AMC parece estar asociada principalmente con la hiperproducción de TEM-1 (sobre todo en E. coli) o con la coexpresión con β-lactamasas tipo OXA-2 y/o SHV (K. pneumoniae y P. mirabilis).
Se describió una nueva variante de blaTEM (TEM-163) en un aislamiento de E. coli que presentó una CIM frente a AMC de 16/8μg/ml. La enzima TEM-163 contiene dos sustituciones de aminoácidos respecto de TEM-1, Arg275Gln y His289Leu. Teniendo en cuenta la alta actividad específica observada y la baja IC50 para el ácido clavulánico, el patrón de resistencia de este aislamiento parece obedecer a la hiperproducción de la nueva variante de la β-lactamasa de amplio espectro, en lugar de vincularse con un comportamiento similar al de una TEM resistente a inhibidores (IRT).
Amoxicillin-clavulanate (AMC) is one of the most frequently prescribed antibiotic combinations in many countries, especially in ambulatory patients. For this reason, even if it is still a low occurrence event, resistance to β-lactam/β-lactamase inhibitors among enterobacteria clinical isolates is an emerging worldwide problem. Different enzymatic mechanisms are associated with AMC resistance: hyperproduction of chromosome-encoded class C β-lactamases, acquired plasmid-encoded cephalosporinases (AmpC type), hyperproduction of plasmid-mediated class A β-lactamases (such as TEM-1 and SHV-1 enzymes), production of class D oxacillinases and inhibitor-resistant TEM (IRTs or CMTs) and SHV mutants6,14,19,23 (whose β-lactamase activities are poorly inhibited by clavulanate). In addition to enzymatic mechanisms, a decrease in the expression or absence of outer membrane proteins might also be involved, i.e., a deficiency in the OmpF and/or OmpC production has been associated to this phenotype in Escherichia coli4.
IRTs were frequently described as plasmid-encoded enzymes derived from TEM-1 or TEM-2 β-lactamases and detected mainly from European isolates. Substitutions of one or more amino-acid residues at positions 69, 244, 275 and 276 render structural changes in β-lactamases that affect affinity for inhibitors, but produce only slight modifications in the isoelectric point (pI) or activity on other β-lactam compounds. Other substitutions also found in IRT β-lactamases do not seem to be involved in the IRT phenotype9.
Overproduction of both TEM-1 and SHV-1, or production of an IRT may increase resistance to amoxycillin, ticarcillin, amoxicillin-clavulanic acid and, frequently to piperacillin and ticarcillin-clavulanic acid, while only slightly affecting susceptibility to narrow spectrum cephalosporins, cephamycins, extended-spectrum cephalosporins, and, in most cases, to piperacillin-tazobactam3.
Over the last years, an increase in the rate of resistance to AMC has been noted among E. coli isolates in our country. According to the “Sistema Informático de Resistencia (SIR)” susceptibility to ampicillin-sulbactam in E. coli has been decreasing from 62%22 (years 2004–2005) to 50% (years 2006–2008; M. Radice, personal communication). In spite of the high use of this association, there is little information available about the prevalence of AMC resistance mechanisms in Enterobacteriaceae.
The aim of this study was to investigate the enzymatic mechanisms of amoxicillin-clavulanate-resistant enterobacteria lacking inducible chromosomal ampC genes isolated from a teaching hospital of Buenos Aires city.
Materials and methodsMicroorganismsIsolates recovered from different clinical specimens that fulfilled a screening criteria (Enterobacteriaceae resistant to ampicillin-sulbactam but remaining susceptible to cefoxitin, ceftazidime and cefotaxime by a disk diffusion method) were studied. Isolates were collected within a 5-month period (May to September 2008) from patients attending the Hospital de Clínicas that belongs to UBA (University of Buenos Aires) “José de San Martín”, Buenos Aires, Argentina.
E. coli ATCC 35218, TEM-1 basal level producer, was used as reference.
Susceptibility testingAntimicrobial susceptibility testing was performed using the disk diffusion method and the agar dilution method as described by the Clinical Laboratory Standard Institute (CLSI). Results were interpreted according to the CLSI guidelines2. Tested antibiotics were ampicillin (AMP), ampicillin-sulbactam (AMS), Amoxicillin-clavulanic acid (AMC), piperacillin (PIP), piperacillin/tazobactam (PTZ), cephalothin (CTN), cefoxitin (FOX), ceftriaxone (CRO), ceftazidime (CAZ), cefepime (FEP), imipenem (IMP), meropenem (MER), ertapenem (ERT), amikacin (AMI), gentamicin (GEN), polymyxin B (POL), trimethoprim-sulfamethoxazole (TMS), ciprofloxacin (CIP), nitrofurantoin (NIT). AMS and AMC MICs were tested at a 2:1 fixed ratio. Antimicrobial agents tested and reported were obtained from Britania S.A. (disks) and Klonal laboratorios (drugs), Argentina.
β-lactamase assaysCells were harvested by centrifugation from overnight LB broth cultures and resuspended in 0.5 ml of sodium phosphate buffer (0.1 M, pH 7). β-lactamases were released by sonication (ten 1-min high-frequency discontinuous bursts on ice at output 4-5 and duty cycle: 50%) using SonicVibra Cell ultrasonic processor (Sonics & Materials Inc., USA).
Isoelectric focusing was performed with crude extracts applied to polyacrylamide gels (pH gradient 3-10) in an LKB 2117-Multiphor II isoelectric focusing tank (LKB Produkter AB, Sweden), as previously described15. β-Lactamase activity was revealed by the agar iodometric system using AMP (500 mg/l) as substrate, as previously described25. TEM-1 (pI 5.4), SHV-2 (pI 7.6), OXA-2 (pI 7.7) and CTX-M-2 (pI 8.2) producing strains were used as standards and were run simultaneously with the samples.
Semi-quantification of β-lactamase production was determined as described below: total protein concentration in crude enzyme preparations was determined by the Bradford method (Bio-Rad Laboratories, USA), adjusted at the same protein concentration and diluted with 100mM sodium phosphate buffer (pH 7.0). β-Lactamase activity of each enzymatic extract and decimal dilutions (20μl) were evaluated using the iodometric method employing AMP as substrate. Clinical isolates that showed β-lactamase activity in dilutions higher than the reference strain were considered enzyme overproducers.
Specific activity was determined in those isolates harboring β-lactamases that focused at pI 5.4. This activity was measured three times per strain by the spectrophotometric assay using nitrocefin (482nm) as substrate at a final concentration of 100μM. TEM-1 overproduction was arbitrarily defined when specific activity was at least two-fold higher than E. coli ATCC 35218.
Inhibition studies were performed by measuring the residual β-lactamase activity of crude extracts previously pre-incubated with clavulanic acid (0.1, 1 and 10μM) for 15min at room temperature. IC50 was defined as the clavulanic acid concentration required to inhibit 50% of the β-lactamase activity.
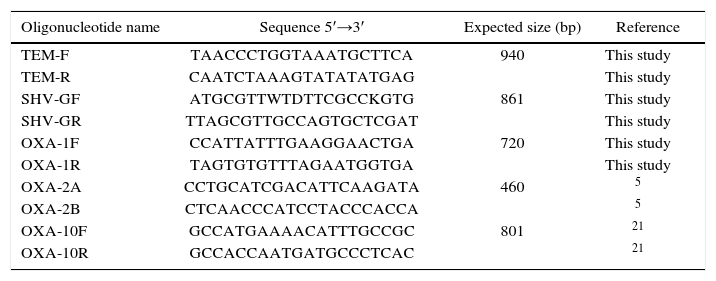
Detection of blaTEM, blaSHV and blaOXA genesMolecular detection of blaTEM, blaSHV and blaOXA-1, blaOXA-2 and blaOXA-10 group was carried out by PCR amplification using specific primers (Table 1). The reaction mixture contained 2μl of total heat extracted DNA as template, and 1.25 U of T-free DNA polymerase (Inbio Highway, Argentina) was added to a total volume of 50μl containing 0.2mM deoxynucleoside triphosphate, 20mM Tris-HCl (pH 8.4), 1.5mM MgCl2, 50mM KCl, and 0.5μM primers. The reaction protocol required an initial step of 5min at 95°C, followed by 30 cycles of 95°C for 1min, 55°C for 1min, and 72°C for 1min, with a final extension at 72°C for 20min. E. coli ATCC 35218, K. pneumoniae ATCC 700603, Salmonella Infantis S215 and Enterobacter cloacae 15321 strains were used as positive controls.
PCR primers used to detect β-lactamase genes and the expected amplicon sizes
| Oligonucleotide name | Sequence 5′→3′ | Expected size (bp) | Reference |
|---|---|---|---|
| TEM-F | TAACCCTGGTAAATGCTTCA | 940 | This study |
| TEM-R | CAATCTAAAGTATATATGAG | This study | |
| SHV-GF | ATGCGTTWTDTTCGCCKGTG | 861 | This study |
| SHV-GR | TTAGCGTTGCCAGTGCTCGAT | This study | |
| OXA-1F | CCATTATTTGAAGGAACTGA | 720 | This study |
| OXA-1R | TAGTGTGTTTAGAATGGTGA | This study | |
| OXA-2A | CCTGCATCGACATTCAAGATA | 460 | 5 |
| OXA-2B | CTCAACCCATCCTACCCACCA | 5 | |
| OXA-10F | GCCATGAAAACATTTGCCGC | 801 | 21 |
| OXA-10R | GCCACCAATGATGCCCTCAC | 21 |
PCR products were purified using either the GFX™ PCR DNA or Gel Band Purification Kit (Amersham Biosciences, Piscataway, NJ, USA). blaTEM sequences were performed on an ABI 377 DNA sequencer (Perkin Elmer, Applied Biosystems). The nucleotide sequences and their derived amino acid sequences were compared to previously described sequences obtained from the GenBank database and β-lactamase classification in Lahey website (http://www.lahey.org/Studies/), respectively. Sequences were aligned using Vector NTI Suite 9 program (InforMax, Inc.).
Molecular epidemiologyThe genetic relationship between the-AMC-resistant E. coli isolates was determined by REP-PCR.
Nucleotide accession numbersThe nucleotide sequence of a novel variant of blaTEM gene (blaTEM-163) was deposited in the GenBank database under accession number EU815939.
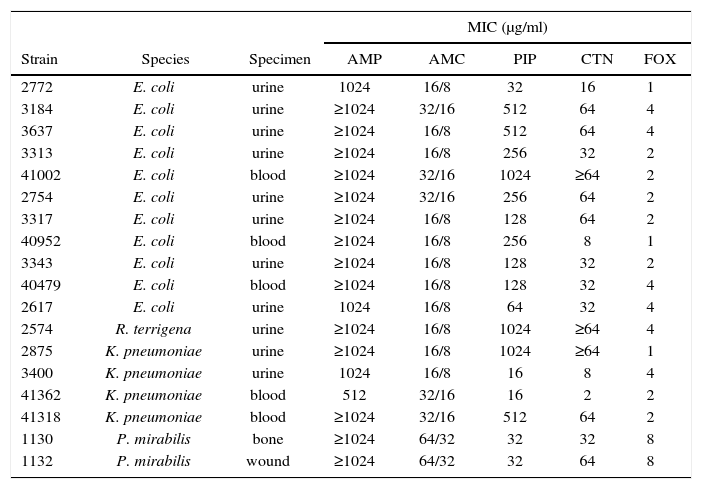
ResultsSusceptibility testingFifty-four (6.4%) out of a total of 843 documented infections were caused by different Enterobacteriaceae that fulfilled the proposed selection criterion. These bacteria, lacking inducible chromosomal AmpC genes, were: 38 E. coli, 8 K. pneumonia, 5 P. mirabilis, 2 K. oxytoca and 1 Raoultella terrigena, and displayed MIC values of AMS higher than 16/8μg/ml.
As not all AMS-resistant isolates are also AMC-resistant16,18; therefore, AMC susceptibility was first determined by the agar disk diffusion method. Taking this feature into account, 18/54 (33.3%) isolates were finally selected to characterize the β-lactamase/s involved: 7 of them were clearly AMC-resistant with inhibition zone diameters <14mm (2 E. coli, 1 R. terrigena, 2 K. pneumoniae and 2 P. mirabilis), 8 had intermediate susceptibility (all E. coli, with inhibition zone diameters between 14-17mm) and 3 had border line susceptibility (1 E. coli and 2 K. pneumoniae, inhibition zone = 18mm).
Resistance profiles were further explored by MIC determination to a large set of antibiotics. Relevant results are shown in Table 2. All of them were sensitive to FOX but PIP and CTN susceptibility was variable. All isolates remained susceptible to PTZ, extended-spectrum cephalosporins (CRO, CAZ, FEP) and carbapenems (IMP, MER, ERT) tested (data not shown). Thus, chromosomal or plasmid AmpC hyper-producing and CMT-producing isolates were ruled out.
Species, specimens and MIC determinations for eighteen selected isolates
| Strain | Species | Specimen | MIC (μg/ml) | ||||
|---|---|---|---|---|---|---|---|
| AMP | AMC | PIP | CTN | FOX | |||
| 2772 | E. coli | urine | 1024 | 16/8 | 32 | 16 | 1 |
| 3184 | E. coli | urine | ≥1024 | 32/16 | 512 | 64 | 4 |
| 3637 | E. coli | urine | ≥1024 | 16/8 | 512 | 64 | 4 |
| 3313 | E. coli | urine | ≥1024 | 16/8 | 256 | 32 | 2 |
| 41002 | E. coli | blood | ≥1024 | 32/16 | 1024 | ≥64 | 2 |
| 2754 | E. coli | urine | ≥1024 | 32/16 | 256 | 64 | 2 |
| 3317 | E. coli | urine | ≥1024 | 16/8 | 128 | 64 | 2 |
| 40952 | E. coli | blood | ≥1024 | 16/8 | 256 | 8 | 1 |
| 3343 | E. coli | urine | ≥1024 | 16/8 | 128 | 32 | 2 |
| 40479 | E. coli | blood | ≥1024 | 16/8 | 128 | 32 | 4 |
| 2617 | E. coli | urine | 1024 | 16/8 | 64 | 32 | 4 |
| 2574 | R. terrigena | urine | ≥1024 | 16/8 | 1024 | ≥64 | 4 |
| 2875 | K. pneumoniae | urine | ≥1024 | 16/8 | 1024 | ≥64 | 1 |
| 3400 | K. pneumoniae | urine | 1024 | 16/8 | 16 | 8 | 4 |
| 41362 | K. pneumoniae | blood | 512 | 32/16 | 16 | 2 | 2 |
| 41318 | K. pneumoniae | blood | ≥1024 | 32/16 | 512 | 64 | 2 |
| 1130 | P. mirabilis | bone | ≥1024 | 64/32 | 32 | 32 | 8 |
| 1132 | P. mirabilis | wound | ≥1024 | 64/32 | 32 | 64 | 8 |
AMP, ampicillin; AMC, amoxicillin-clavulanic acid; PIP, piperacillin; CTN, cephalothin; FOX, cefoxitin.
Regarding susceptibility to non β-lactam antibiotics, 9/11 E. coli were resistant to TMS, 4/11 to CIP and 3/11 to NIT; 4/4 K. pneumoniae were resistant to TMS and NIT, 2/4 were resistant or had intermediate susceptibility to GEN and AMK, and 1/4 was resistant to CIP; and finally 2/2 P. mirabilis were resistant to GEN and NIT, and 1/2 was resistant to CIP. Except for natural resistance in P. mirabilis, all isolates were susceptible to POL.
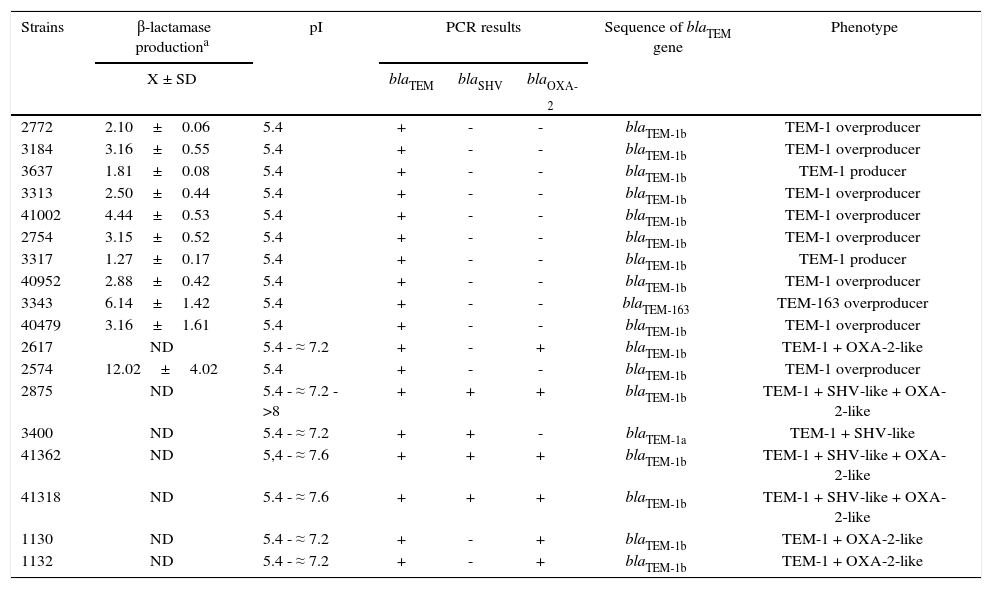
Resistance mechanismsAll crude extracts showed β-lactamase activity when analyzed by the iodometric assay employing AMP as substrate. Characterization of the β-lactamases revealed that all extracts possessed, at least, an enzyme that focused at pI 5.4 and 7/18 co-produced other enzymes that focused at different pI (Table 3). Ten of eleven E. coli isolates only displayed the pI 5.4 enzyme.
Characterization of β-lactamases produced by studied isolates
| Strains | β-lactamase productiona | pI | PCR results | Sequence of blaTEM gene | Phenotype | ||
|---|---|---|---|---|---|---|---|
| X ± SD | blaTEM | blaSHV | blaOXA-2 | ||||
| 2772 | 2.10±0.06 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 3184 | 3.16±0.55 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 3637 | 1.81±0.08 | 5.4 | + | - | - | blaTEM-1b | TEM-1 producer |
| 3313 | 2.50±0.44 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 41002 | 4.44±0.53 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 2754 | 3.15±0.52 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 3317 | 1.27±0.17 | 5.4 | + | - | - | blaTEM-1b | TEM-1 producer |
| 40952 | 2.88±0.42 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 3343 | 6.14±1.42 | 5.4 | + | - | - | blaTEM-163 | TEM-163 overproducer |
| 40479 | 3.16±1.61 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 2617 | ND | 5.4 - ≈ 7.2 | + | - | + | blaTEM-1b | TEM-1 + OXA-2-like |
| 2574 | 12.02±4.02 | 5.4 | + | - | - | blaTEM-1b | TEM-1 overproducer |
| 2875 | ND | 5.4 - ≈ 7.2 - >8 | + | + | + | blaTEM-1b | TEM-1 + SHV-like + OXA-2-like |
| 3400 | ND | 5.4 - ≈ 7.2 | + | + | - | blaTEM-1a | TEM-1 + SHV-like |
| 41362 | ND | 5,4 - ≈ 7.6 | + | + | + | blaTEM-1b | TEM-1 + SHV-like + OXA-2-like |
| 41318 | ND | 5.4 - ≈ 7.6 | + | + | + | blaTEM-1b | TEM-1 + SHV-like + OXA-2-like |
| 1130 | ND | 5.4 - ≈ 7.2 | + | - | + | blaTEM-1b | TEM-1 + OXA-2-like |
| 1132 | ND | 5.4 - ≈ 7.2 | + | - | + | blaTEM-1b | TEM-1 + OXA-2-like |
All isolates were positive for blaTEM and PCR products were sequenced subsequently. Variants of the blaTEM-1 gene were detected, being blaTEM-1b described in 16 isolates. The blaTEM-1a allele was only noted in one K. pneumoniae strain (Table 3) and no other variants of blaTEM-1 gene were detected. An E. coli isolate (strain 3343) produced a new variant of the blaTEM gene (named blaTEM-163), which differed from blaTEM-1b in 3 bp (see below for more details).
When tested for other β-lactamase genes by PCR, only one E. coli, three K. pneumoniae and two P. mirabilis isolates were positive for blaOXA-2-group genes (Table 3). The blaSHV gene was only detected in all K. pneumoniae isolates. Not a single isolate rendered a positive amplification for blaOXA-1 or blaOXA-10 group. These results agreed with pI values previously obtained.
Most isolates that only harbored the blaTEM-1 determinant (7 E. coli and 1 R. terrigena) showed a higher level of TEM-1 production, and could be considered as probable TEM-1 overproducers since they showed an enzyme production 2-12-fold higher than E. coli ATCC 35218 (Table 3). The remaining two E. coli strains, with intermediate susceptibility to AMC, displayed a basal or slightly increased level of TEM-1, suggesting that other mechanisms would be involved in the susceptibility decrease observed. Results achieved by the spectrophotometer method were coincident with those obtained by the iodometric semi-quantitative assay.
E. coli 3343, in which the new TEM-163 was present, also showed a profile compatible with TEM overproduction (with a specific activity that was 6-fold higher than E. coli ATCC 35218). In this case, IC50 values for clavulanic acid were similar to the IC50value measured on the reference TEM-1-producing strain (IC50 around of 1μM). Because of these characteristics, rather than thinking of TEM-163 as a probable IRT β-lactamase, the AMC-resistance phenotype is likely due to overproduction of this new β-lactamase.
As expected from isolates that were mostly obtained from urine samples with no strong epidemiological link, even those producing only a TEM β-lactamase, the different E. coli isolates are assumed as the different clones displayed a dissimilar banding profile by REP-PCR (data not shown).
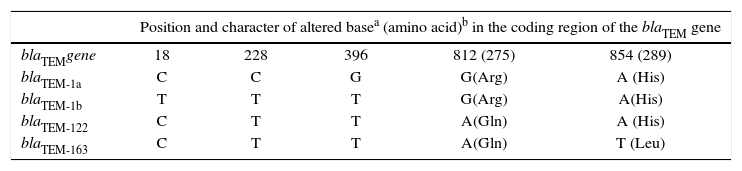
TEM-163 versus TEM-122TEM-163-producing E. coli was resistant to AMP, AMS (MIC≥32/16μg/ml), PIP and CTN, of intermediate susceptibility to AMC (MIC=16/8μg/ml) and susceptible to FOX (Table 2). The blaTEM-163 gene presents more than 99% identity with blaTEM-122 (previously considered IRT)10. blaTEM-122 variant (accession number AY307100) could be considered a blaTEM-1b-derived gene that has a G→A transition at base 812, leading to Arg→Gln amino acid change at position 275 (numbering according to Ambler et al.1). A new mutation (A→T transversion) at base 854 was detected in blaTEM-163 producing a His→Leu amino acid substitution at position 289 in the mature protein of TEM-163, which has not been previously reported (Table 4). Distinctiveness of these mutations was verified by sequencing both the coding and non-coding strands from two independent PCR reactions.
Nucleotide mutations and amino acid substitutions in E. coli isolate encoding new β-lactamase
| Position and character of altered basea (amino acid)b in the coding region of the blaTEM gene | |||||
|---|---|---|---|---|---|
| blaTEMgene | 18 | 228 | 396 | 812 (275) | 854 (289) |
| blaTEM-1a | C | C | G | G(Arg) | A (His) |
| blaTEM-1b | T | T | T | G(Arg) | A(His) |
| blaTEM-122 | C | T | T | A(Gln) | A (His) |
| blaTEM-163 | C | T | T | A(Gln) | T (Leu) |
The sequences are compared to those of blaTEM-1a and blaTEM-1b.
We have focused the study on β-lactamases present in different Enterobacteriaceae with phenotypic resistance to β-lactam/β-lactamase inhibitors but remaining susceptible to cefoxitin and extended-spectrum cephalosporins. During the 5-month period of our study the overall frequency of ampicillin-sulbactam-resistant isolates (all non-inducible AmpC producing enterobacteria) remained at about 6.4%. Among them, we selected those that also showed a reduced inhibition zone to AMC by the disk diffusion test to describe the enzymatic mechanisms that are poorly known in our country. AMC resistance in Enterobacteriaceae is a complex phenomenon with heterogeneous clinical implications.
The overproduction of TEM-like enzymes followed by TEM-1 production in combination with OXA-2-type and/or SHV-type β-lactamases were the main mechanisms identified.
In agreement with literature data, genes encoding TEM-1 were detected in the majority of the isolates examined, being blaTEM-1b the predominant allelic variant (16/18) identified so far, and only one blaTEM-1a harboring strain (K. pneumonia 3400) was described. Consistently, other blaTEM-1 variants (i.e. blaTEM-1c, blaTEM-1d, blaTEM-1e), rarely detected in enterobacteria13,17, were not found.
The presumed overproduction of TEM-1 β-lactamase in E. coli, which was present in 7/11 isolates with reduced susceptibility to AMC, was the main mechanism responsible for that resistance profile. These data are similar to those detected in E. coli by Pérez-Moreno et al. in Spain20, although other researchers have observed a lower proportion19,24.
At least two mechanisms may lead to TEM overproduction: 1- multiple copies of the blaTEM gene due to the presence of multiple copies of a plasmid per cell have been reported to cause overproduction of β-lactamase or, 2- different promoter regions have been widely reported in various isolates. Even though blaTEM-1a and blaTEM-1b alleles were initially associated with a weak promoter7 (called P3), it is known that stronger promoters could be present (i.e., Pa/Pb, P4, P5) upstream of blaTEM-like genes, which would explain the higher levels of production of TEM enzymes11–13.
Two E. coli isolates producing a basal or slightly increased level of TEM-1 as a single resistance mechanism showed reduced susceptibility to AMC and CTN, being the reduced permeability or increased efflux to β-lactam antibiotics the possible resistance mechanisms involved4,20.
In the present study, OXA-2-type enzymes were mainly associated with K. pneumoniae and P. mirabilis, while the OXA-1 and OXA-10 groups were not detected. OXA-2-like enzymes, known to be more poorly inhibited by clavulanic acid than class A β-lactamases, accounted for 33% (6/18) of the isolates analyzed, but when E. coli determinants were compared with other studies13,19,20,24, the frequency of OXA-producing E. coli remained low (1/11).
Genes encoding SHV-1 and other close related enzymes have been shown to be natural in K. pneumoniae chromosome9; therefore blaSHV detection in this species is always expectable.
Contribution of IRT β-lactamases to AMC resistance in Enterobacteriaceae appear to be different in isolates from diverse countries. In this study, IRT-like enzymes were not detected among isolates having intermediate susceptibility and also among those that were clearly resistant. To the best of our knowledge, the presence of IRT-producing isolates appears to be much less common in the American continent than in Europe14. Nevertheless, amplification and direct sequencing of blaTEM has contributed to discover a new enzymatic variant called TEM-163 present in an E. coli strain having intermediate susceptibility to AMC (MIC= 16/8 mg/l). The predicted amino acid sequence of the latter contained the same substitution present in TEM-12210 (Arg275Gln), and also provided an extra mutation (His289Leu), which has not been previously described.
An in silico model of TEM coevolution network and its communities was constructed by Guthrie et al., and the two mutant positions described in this work (275, 289) are included within the inhibitor-resistant β-lactamase community. Interestingly, position 289 is located at the interface between the two communities8 (extended-spectrum and inhibitor resistance).
The amino acid substitution at position 275 (located at the C-terminal of the α-11 helix) was previously considered important to confer resistance to β-lactamase inhibitors. However, the same substitution (Arg275Gln) was involved in TEM-45 (IRT-14), TEM-82 and TEM-83 but these enzymes contained at least a second mutation in residue 6910,13, which was highly associated with IRT enzymes3,9.
The absence of a mutation in residue 69 on this new β-lactamase in agreement with its low IC50 to clavulanic acid allow us to speculate that TEM-163 is more clearly a new variant of broad spectrum β-lactamase than an IRT enzyme (IC50>10μM is considered a probable IRT enzyme20). In conclusion, amoxicillin-clavulanate resistance in different species of non-inducible AmpC Enterobacteriaceae isolated in a teaching hospital from Buenos Aires seems to be mainly associated with TEM-1 overproduction or TEM-1, OXA-2-like and/or SHV-like β-lactamase co-production.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was partly supported by UBA and ANPCyT grants to G.O.G., UNL and ANPCyT grants to J.A.D.C and from the Ministerio de Ciencia e Innovación, Spain (BFU2009-09200/BMC) to J.A.A. J.A.D.C. and G.O.G. are members of the Carrera del Investigador Científico, CONICET, Argentina.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank Ms. Ruggiero M. and Ms. Dabos L. for their help in kinetic determinations.