The aim of this work was to determine the effects of two geographically different strains of Rhizophagus intraradices (M3 and GA5) on the total biomass and essential oil (EO) yield and composition of Calamintha nepeta, with or without phosphorus (P) fertilization, under greenhouse conditions. The plant biomass was not significantly affected by any of the treatments, showing higher values in control plants. Strains had a differential response in their root colonization rates: M3 reduced these parameters while GA5 did not modify them. Both strains affected EO yield in absence of P fertilization: M3 promoted EO yield in C. nepeta plants and GA5 resulted in negative effects. The percentage composition of EO was not significantly modified by either strain or P fertilization. M3 strain could be a potential fungal bioinoculant for production and commercialization of C. nepeta in the aromatic plant market.
El objetivo de este trabajo fue determinar, bajo condiciones de invernadero, el efecto de dos cepas geográficamente diferentes de Rhizophagus intraradices (M3 y GA5) sobre la biomasa total y el rendimiento y composición de aceites esenciales (AE) de Calamintha nepeta, con fertilización fosforada (P) o sin esta. La biomasa de la planta no fue significativamente afectada por ningún tratamiento, y se observaron valores más altos en las plantas control. Las cepas mostraron diferencias en sus tasas de colonización y en las respuestas a la fertilización con fósforo: M3 redujo sus valores de colonización, mientras que GA5 no los modificó. En ausencia de fertilización fosforada, las plantas colonizadas por ambas cepas presentaron rendimientos de AE diferentes a aquellos de las plantas control: M3 los aumentó y GA5 los disminuyó. La composición porcentual de AE no fue modificada significativamente por ninguno de los tratamientos. M3 podría ser considerada como un posible bioinoculante fúngico para la producción de C. nepeta destinada al mercado de las plantas aromáticas.
Calamintha nepeta subsp. nepeta (Lamiaceae), which is native to the Mediterranean basin, is an introduced species of aromatic plants in Argentina. It is a domesticated plant used for herbal infusions10 under the same commercial name of the native species Minthostachys molli, ‘peperina’, due to their similar flavor and aroma15. M. molli is nowadays becoming increasingly scarce because of its overexploitation14. The essential oils (EO) of aromatic plants such as C. nepeta, are volatile lipophilic mixtures of secondary plant compounds, mainly used in food, cosmetic and pharmaceutical industries2. EO yield is strongly influenced by biotic and abiotic factors6.
It has been shown that arbuscular mycorrhizal (AM) symbiosis induces changes in secondary compounds which act as signal molecules in plant-AM fungal interaction12. Previous studies have been focused on different AM fungal effects on several aromatic plants: biomass production7,13, EO yield and composition6 and nutrient uptake7,13. It has been demonstrated that different species and isolates of AM fungi have diverse effects on mycorrhizal plants and that different plant species or varieties react differently to the same AM isolates8. However, to our knowledge there are no reports about the potential effect of AM mycorrhization on EO yield and composition in C. nepeta plants.
The purpose of this work was to evaluate the effect of two geographically different strains of R. intraradices on plant growth response and EO yield and composition of C. nepeta, with or without phosphorous (P) fertilization, under greenhouse conditions. We also aim at improving the quality and quantity of the EO of C. nepeta in order to promote its commercial production, protecting the endangered native plant species.
A C. nepeta plant used for the assay was collected in Jujuy province, Argentina. A single healthy and vigorous plant of C. nepeta was chosen. Cuttings (10cm) were collected from the mother plant in order to avoid genetic variability in the experiment. Cuttings were established in sterile perlite until the differentiation of adventitious roots. Rooted cuttings were transplanted [1 plant per 500 ml pot with 300 ml autoclaved substrate (100 °C for 1 h, three consecutive days)] into a mixture of soil:perlite (1:2) as growth substrate. The soil used had the following features: clay loam soil; pH 7.1; total C: 12.08 and N: 1.1 (g/Kg); P: 34.2 (mg/Kg); K: 0.9, Ca 7.5,Mg: 1.7 and Na: 0.2 (cmol/Kg).
Each plant was placed in a hole where the inoculum had been previously added. The inoculum, consisted of colonized roots of Trifolium sp. (over 70% of AM fungal colonization) and soil with hyphae and spores, previously quantified in order to use the same amount for each pot (approx. 50 spores per plant). Two different strains of R. intraradices were used in this experiment: GA5 [Banco de Glomeromycota in Vitro (BGIV) collection of the Laboratorio de Microbiología del Suelo, Facultad de Ciencias Exactas y Naturales, UBA, (http://www.bgiv.com.ar/strains/Rhizophagusintraradices/ga5)] and M3 [collection of the Laboratorio de Microbiología del Suelo, Facultad de Ciencias Exactas y Naturales, UBA]. The latter strain was isolated from the same geographic region of C. nepeta.
Pots were grown in a random design for 60 days in a greenhouse with controlled temperature and humidity conditions at the Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. All the plants were watered daily. Ten milliliters (10ml) of PO4H2Na (0.05g/l) were added to the corresponding treatment once a week. The P solution concentration is consistent with that routinely used in Hewitt's nutrient solution5. Treatments were: 1- C (Control): perlite-sterilized soil substrate; 2- CP: perlite-sterilized soil substrate and P fertilization; 3- M3: perlite-sterilized soil substrate inoculated with M3; 4- M3P: perlite-sterilized soil substrate inoculated with M3 and P fertilization; 5- GA5: perlite-sterilized soil substrate inoculated with GA5; 6- GA5P: perlite-sterilized soil substrate inoculated with GA5 and P fertilization. Three replicates (each replicate was composed by ten pots) were performed for each treatment.
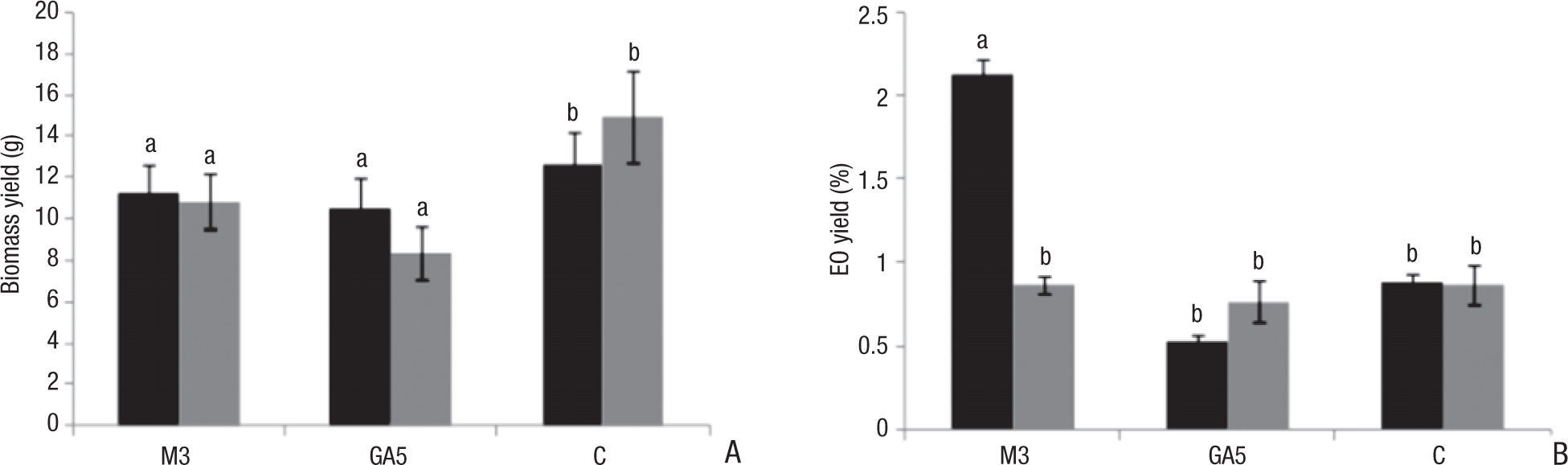
Root samples were taken from each treatment after 30 days of growth to evaluate the success of mycorrhizal colonization. After 60 days of growth, plants were harvested and air-dried in a dark room for their final processing and measurement of biomass yield. Biomass was significantly higher in control plants than in mycorrhized ones (F=12.822, p<0.05), particularly when P fertilization was performed (C: 12.65g, CP: 14.96g). Mean values correspond to plants inoculated with M3 strains (M3: 11.28g, M3P: 10.85g), while the lowest biomass yields were those of plants colonized by GA5, especially when P fertilization was performed (GA5: 10.47g, GA5P: 8.33g). Nevertheless, the observed differences between strains resulted nonsignificant when they were statistically analyzed (Fig. 1A).
Effect of phosphorus fertilization and inoculation of arbuscular mycorrhizal strains, M3 and GA5, on: (A) biomass yield, and (B) essential oil yield, in Calamintha nepeta. C represents values for control plants. Black bars correspond to non-phosphorus fertilized treatments. Grey bars correspond to phosphorus-fertilized treatments. Mean values with error bars. Different letters represent significant differences (p < 0.05). EO: essential oil.
AM fungal colonization was also evaluated at this time. Root samples were cleared in KOH (10%) for 24 h at room temperature and then stained with trypan blue in lactic acid (0.02%) for 24 h at room temperature (modified procedure of Phillips and Hayman's technique11). Mycorrhization was detected in all treatments, except in control plant roots. Intraradical colonization was quantified from 60 randomly selected root segments (1cm long) per treatment. Frequency (%F) and intensity (%I) of mycorrhizal colonization was calculated according to Declerck et al.3 A Nikon light binocular microscope (model: Optiphot-2) was used for this purpose.
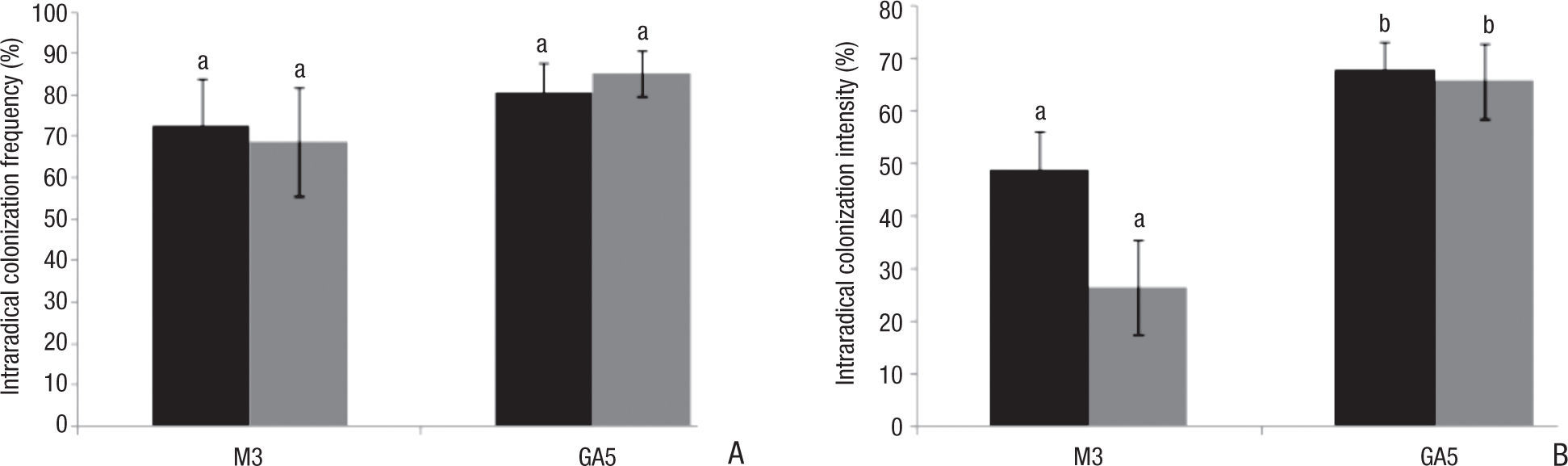
Our results showed that P fertilization had different effects on root colonization by both R. intraradices strains tested. Mycorrhization frequency (Fig. 2A) reached values above 70% but no significant differences between treatments were found (F=0.355, p=0.556). The GA5 strain showed the highest colonization frequency, particularly when P fertilization was performed (GA5: 80.65%; GA5P: 85.35%). P-fertilized roots inoculated with the M3 strain presented the lowest mycorrhization frequency (M3: 72.50%; M3P: 68.75%). Mycorrhization intensity (Fig 2B) resulted significantly higher in plants inoculated with GA5 (F=29.361, p<0.05), especially when plants were not P-fertilized (GA5: 67.69%; GA5P: 65.70%). The lowest intensity percentages corresponded to P-fertilized plants inoculated with the M3 strain (M3: 48.78%; M3P: 26.42%), coinciding with mycorrhization frequency results. As expected, the intensity of root colonization by M3 decreased with P fertilization; however this difference was not observed in GA5 treatments.
Colonization of Calamintha nepeta roots by arbuscular mycorrhizal strains GA5 and M3 expressed as: (A) percentage (%) of intraradical colonization frequency, and (B) percentage (%) of intraradical colonization intensity. Black bars correspond to nonphosphorus fertilized treatments. Grey bars correspond to phosphorus-fertilized treatments. Mean values with error bars. Different letters represent significant differences (p < 0.05).
Ryan et al. observed that P levels in soil and host plant were responsible for the degree of root colonization by AM fungi. It is possible that genetic variations between R. intraradices strains could result in significant functional variations8 and, therefore, in a differential response to P fertilization.
In the present study, we observed that the lowest value of plant biomass coincided with the highest level of AM fungal colonization. This may be a negative effect of the inoculation of GA5 on C. nepeta plants. Similar results were observed by Koch et al.8 when two genetically different R. intraradices strains negatively influenced the growth of Daucus carota transformed roots. These authors related this observation to the cost of AM fungal colonization for the plant8.
After natural drying, the plant stems were subjected to hydrodistillation for 3 h using a Clevenger-type trap. Extracted essences were dried with anhydrous sodium sulphate and stored in vials at -20 °C for qualitative and quantitative analysis. EO yield of C. nepeta plants was calculated as: (EO volume / Plant dry weight) x 1001.
M3 strain significantly promoted EO yield (2.13%, F=43.346, p<0.05) (Fig 1B). However plants inoculated with this strain and P-fertilized showed similar values to those of control plants (0.87%). The highest value coincided with the lowest frequency and intensity of AM fungal colonization. Otherwise, the lowest yield was obtained when C. nepeta was inoculated with GA5 (0.53%); these plants presented the highest values of fungal colonization.
Our results suggested a positive correlation between the level of M3 colonization and EO yield in C. nepeta. The opposite correlation was observed when GA5 was inoculated. Since it is known that different strains of R. intraradices have either favorable or harmful effects in host plant development8, this could be a reasonable explanation for the results in this study.
For qualitative and quantitative analysis of the EO, hyphenated gas chromatography with a flame ionization detector and mass spectrometer (GC-FID-MS) was performed on a Perkin Elmer Autosystem gas chromatograph (model: Clarus 500). GC conditions: Mobile phase: Helium 1.87 ml/ min; autosampler connected to a single injector split (split relation: 1:100) connected by a flow splitter to two fused silica capillary columns (5% fenil-95% dimethylpolysiloxane and polyethyleneglycol 20 000, both of 60 m x 0.25 μm with a film thickness of 0.25 μm); temperature program: 50 °C, then 3 °C/min until 225 °C (15 min); injector and detector temperature FID: 255 °C and 275 °C, respectively; transfer line temperature: 180 °C; ion source temperature: 150 °C; total run time: 70 min; scanned mass range: 40-400 m/z; injected volume: 0.2 μl of a 10% dilution in ethanol.
EO constituents were identified on the basis of their retention index and their MS, which were compared with those found in literature data and standards of the Cátedra de Farmacognosia (Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires). The percentage composition was determined by the percentage of areas of each peak, without the correction factor. The lowest response for each component was taken among those obtained from each column used. A homologous series of methyl esters of fatty acids (C4-C18) was co-chromatographed with the oil for determination of the retention index.
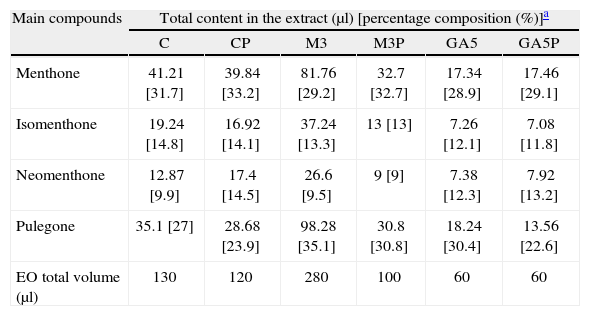
The main compound contents in the EO of C. nepeta are shown in Table 1. Even when the total contents of each main compound seem to differ among treatments, these differences were not statistically significant. The percentage of menthone was always higher when P fertilization was performed with respect to the same treatments without P supplementation (e.g. C: 31.7%; CP: 33.2%). The opposite was observed with isomenthone (e.g. C: 14.8%; CP: 14.1%) and pulegone (e.g. C: 27%; CP: 23.9%). Finally, neomenthol followed the menthol tendency, except for treatments involving M3 inoculation. Menthone and isomenthone reached their highest values in control plants, while the percentage composition of neomenthol and pulegone was improved by GA5 and M3 inoculation respectively. These percentage changes in EO composition resulted in a variation in the menthone/pulegone ratio. While nonmycorrhized and mycorrhized plants with P supplementation had a menthone/pulegone ratio greater than 1, the inoculated ones with no P supplementation presented a value lower than 1.
Total content (μl) of each main compound in the essential oil extracted from Calamintha nepeta according to the different treatments
| Main compounds | Total content in the extract (μl) [percentage composition (%)]a | |||||
| C | CP | M3 | M3P | GA5 | GA5P | |
| Menthone | 41.21 [31.7] | 39.84 [33.2] | 81.76 [29.2] | 32.7 [32.7] | 17.34 [28.9] | 17.46 [29.1] |
| Isomenthone | 19.24 [14.8] | 16.92 [14.1] | 37.24 [13.3] | 13 [13] | 7.26 [12.1] | 7.08 [11.8] |
| Neomenthone | 12.87 [9.9] | 17.4 [14.5] | 26.6 [9.5] | 9 [9] | 7.38 [12.3] | 7.92 [13.2] |
| Pulegone | 35.1 [27] | 28.68 [23.9] | 98.28 [35.1] | 30.8 [30.8] | 18.24 [30.4] | 13.56 [22.6] |
| EO total volume (μl) | 130 | 120 | 280 | 100 | 60 | 60 |
EO, essential oil.
P fertilization had no influence in EO yield or composition in plants without fungal colonization. The supplementation of this nutrient, as performed in the present study, was not a limiting factor for these variables in C. nepeta. Our study partially supports Hammer et al.4 results, where P fertilization does not change the physical and chemical properties of EO in C. nepeta, but, the pulegone content is increased at high P rates. Nell et al.9 concluded that P fertilization improves biomass yield and secondary metabolite concentration in Salvia officinalis (garden sage), reaching higher values than AM-inoculated plants. Phosphorus is an important constituent of nucleic acids and phospholipids. For this reason, plants required it in large amounts for biosynthesis of primary and secondary metabolites. Besides P fertilization, AM fungal symbiosis positively affects the P status of the plant9. In this context we expected that AM inoculation and P fertilization would have similar effects on EO yield and composition in C. nepeta. Although our results differ from those of Hammer et al.4 and Nell et al.9, further experiments with higher rates of P fertilization should be performed.
Two-way ANOVA was performed for all data. Treatment effects were analyzed using the Tukey HSD test (p < 0.05). Homogeneity of variances and normal distribution assumptions were checked with the Levene test and distribution of within-self residuals, respectively. All statistical analyses were performed with STATISTICA, version 7 StatSoft, Inc. (2004).
To our knowledge, this is the first study on the effect of AM fungal inoculation in C. nepeta. Further studies with more species and strains of AM fungi on this host plant are necessary to find out possible mutualistic interactions that promote both EO and dry biomass yields. The effect of AM fungi colonization on the EO of aromatic plants was studied in different host plants and, in every case, the response to colonization was different2,6,12,13.
According to the interactions observed between the two fungal strains tested and C. nepeta plants, we conclude that some strains of R. intraradices, in this case the M3 strain, could be used as bioinoculants in C. nepeta production due to their positive inoculation effect on EO yield and non-modification on fragrance properties, without affecting total biomass. Furthermore, the commercialization of M. molli ‘peperina’ in the aromatic plants market is done by potting10, thus facilitating AM fungi inoculation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We wish to thank UBA, CONICET and ANCYPT for financial support. We also acknowledge Dra. Vanesa Silvani (Facultad de Ciencias Exactas y Naturales, UBA, Argentina), Dr. Arnaldo Bandoni (Cátedra de Farmacognosia-IQUIMEFA, UBA, Argentina), and Dr. Alexander N. Schmidt-Lebuhn (Institut für Systematische Botanik, Zurich, Switzerland), for their technical assistance.