Plant growth promoting microorganisms play a substantial role in current plant production practices. Yeasts have shown a promising role in enhancing plant production. We studied the production of indole 3-acetic acid (IAA)-like compounds in three psychrotolerant Patagonian native yeast strains. The yeast Tausonia pullulans CRUB 1772, Candida saitoana CRUB 1770 and Saccharomyces eubayanus CRUB 2014 were evaluated using the Salkowski's colorimetric technique and high-performance liquid chromatography (HPLC). T. pullulans CRUB 1772 was the highest producer of IAA-like compounds using the colorimetric technique. Nevertheless, none of the tested yeasts reached the production level of the bacterial strain A. argentinense Az39 used as positive control. The HPLC analysis revealed that only C. saitoana CRUB 1770 and S. eubayanus CRUB 2014 were able to truly produce IAA. Other peaks were also observed, which might correspond to intermediate compounds within the IAA biosynthetic pathway.
Los microorganismos promotores del crecimiento vegetal juegan un papel importante en las prácticas de producción vegetal actuales. Las levaduras han mostrado un rol prometedor para mejorar la producción vegetal. En este trabajo se investigó la producción de compuestos tipo ácido indol-3-acético (AIA) en 3 levaduras psicrotolerantes nativas de la Patagonia: Tausonia pullulans CRUB 1772, Candida saitoana CRUB 1770 y Saccharomyces eubayanus CRUB 2014. Para ello se utilizó la técnica colorimétrica con el reactivo de Salkowski y cromatografía líquida de alto rendimiento. El mayor productor de compuestos tipo AIA por colorimetría fue T. pullulans CRUB 1772. Sin embargo, ninguna de las levaduras alcanzó los niveles de producción de la bacteria Azospirillum argentinense Az39, utilizada como control positivo. El análisis por cromatografía líquida de alto rendimiento reveló que solo C. saitoana CRUB 1770 y S. eubayanus CRUB 2014 fueron capaces de producir AIA. También se observaron otros picos, que podrían corresponder a compuestos intermediarios de la vía biosintética del AIA.
Plant growth promoting microorganisms (PGPM) arise as a biotechnological tool to reduce the use of chemical fertilizers and enhance food production, when searching for sustainable practices in agriculture. Several reports support a promising role of yeasts as PGPM due to the production of plant hormones and other growth regulators1,11,12,15. Phytohormones, such as auxins, have been associated with enhanced root growth leading to improved water and nutrient uptake by plants, with indole 3-acetic acid (IAA) being the most found natural auxin4. Moreover, auxin production has been studied as PGPM marker for yeasts, and the reports include a variety of species within Ascomycota and Basidiomycota Phyla such as Candida tropicalis1, Meyerozyma guillermondi8, Williopsis saturnis and Rhodotorula glutini11, Torulaspora globosa and Rhodotorula mucilaginosa2.
In recent years, many yeast species were isolated from Nothofagus native forests in Patagonia9. In these cold-temperate woods, the reported yeast community included species which were not commonly reported in PGPM studies involving yeasts. Several Patagonian yeasts were characterized as potential plant growth promoters due to their ability to produce auxin-like compounds9,10. The potential of these yeasts is worth mentioning, since in cold-temperate regions, the use of cold-adapted or psychrotolerant yeasts with plant growth promoting features could be an attractive strategy to promote sustainable agriculture. In the present study, we explored the production of the auxin IAA as a plant growth promoting feature produced by Tausonia pullulans CRUB 1772, Candida saitoana CRUB 1770 and Saccharomyces eubayanus CRUB 2014 isolated from soil in northwestern Patagonia10. These three yeast strains are part of the Yeast Collection of the Centro Regional Universitario Bariloche, Universidad Nacional del Comahue (Argentina), and have previously been proposed as plant growth promoters9,10.
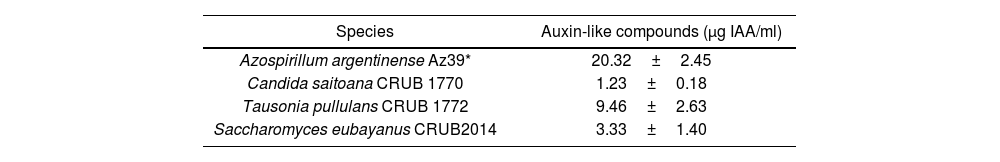
Colorimetric detection and quantitative assessment of IAA-like compounds was performed in supernatant from liquid cultures in DEV+glu medium (NaCl 5g/l, soy peptone 10g/l, glucose 5g/l) with 0.1% tryptophan, using three biological replicates10. All cultures were incubated for 7 days at 20°C, and later centrifuged to recover the supernatants. Detection of IAA-like compounds was performed using Salkowski's reagent (FeCl3 12g/l, H2SO4 7.9M) followed by absorbance determination at 530nm in an EPOCH 2 Microplate Reader (Biotek). The quantification of IAA-like compounds was performed by the external standard calibration technique using a commercial IAA (Sigma) and informed as IAA equivalents (μg IAA/ml). Therefore, IAA was used as a proxy for auxin production. Azospirillum argentinense Az39, formerly Azospirillum brasilense Az393, was used as a positive control. The colorimetric determination rendered positive results for all yeast strains, although the concentration produced was variable (Table 1). When compared with A. argentinense Az39, the production of IAA-like compounds by yeasts was lower (two to ten times lower). The highest IAA-like compound production was registered for T. pullulans CRUB 1772 (9.46±2.63μg IAA/ml).
Detection of auxin-like compound production by colorimetric techniques using Salkowski's reagent.
| Species | Auxin-like compounds (μg IAA/ml) |
|---|---|
| Azospirillum argentinense Az39* | 20.32±2.45 |
| Candida saitoana CRUB 1770 | 1.23±0.18 |
| Tausonia pullulans CRUB 1772 | 9.46±2.63 |
| Saccharomyces eubayanus CRUB2014 | 3.33±1.40 |
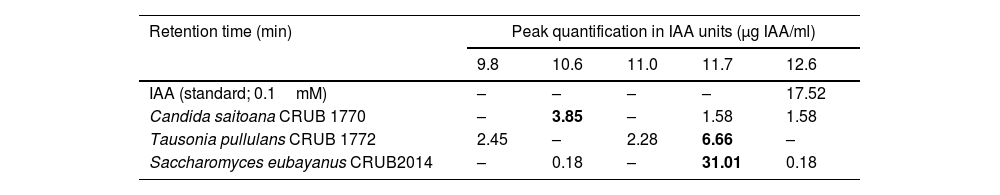
For the detection of IAA by high-performance liquid chromatography (HPLC), the three strains were cultivated in liquid DEV+glu medium (NaCl 5g/l, soy peptone 10g/l, glucose 5g/l) with 0.1% tryptophan for 7 days at 20°C, using three biological replicates. All cultures were centrifuged to recover the supernatant and filtered with 0.22μm nylon membrane prior to injection into the HPLC system. The HPLC equipment consisted of a multisolvent delivery system (Waters 600E) with a photodiode array detector (Waters 2998), controlled with Empower 2 software. Separation was performed at room temperature, using a RP-C18 column (Phenomenex), 25cm×4.6mm and particle size 5μm. The mobile phase was water/methanol/acetic acid (70:29:1) v/v at a flow rate of 0.8ml/min and the volume injected of each filtered culture was 40μl. The eluted compounds were monitored at 280nm, and UV spectra (200–500nm) were recorded for peak characterization. Quantifications were performed using the external standard method and IAA (Chemlm Ltd.) standards, with concentrations ranging from 0.01mM to 0.50mM. The quantification of every peak was performed with the same calibration curve and were referred as IAA units in μg IAA/ml (MWIAA=175.19g/mol). The HPLC detection method revealed the presence of five distinct peaks (Table 2). The peak of IAA had a retention time of 12.6min. This peak was observed in the supernatant of C. saitoana CRUB1770 and S. eubayanus CRUB2014 but was missing in T. pullulans CRUB1772 supernatant. Moreover, the three yeast strains shared the presence of a peak at 11.7min; C. saitoana CRUB1770 and S. eubayanus CRUB2014 shared a peak at 10.6min while T. pullulans CRUB1772 supernatant was the only one that exhibited peaks at 9.8 and 11.0min.
Detection of indole 3-acetic acid (IAA) in three yeast strains by HPLC.
| Retention time (min) | Peak quantification in IAA units (μg IAA/ml) | ||||
|---|---|---|---|---|---|
| 9.8 | 10.6 | 11.0 | 11.7 | 12.6 | |
| IAA (standard; 0.1mM) | – | – | – | – | 17.52 |
| Candida saitoana CRUB 1770 | – | 3.85 | – | 1.58 | 1.58 |
| Tausonia pullulans CRUB 1772 | 2.45 | – | 2.28 | 6.66 | – |
| Saccharomyces eubayanus CRUB2014 | – | 0.18 | – | 31.01 | 0.18 |
–: undetected peak.
Values in bold correspond to the highest concentration in each sample.
In our study, the production of IAA-like compounds was observed for all the tested yeasts, although their production levels were lower than those observed for A. argentinense Az39 under the same culture conditions. A similar trend was reported by Amprayn et al.1, who observed that the A. brasilense sp245 strain showed higher IAA production (30μg IAA/ml) than that of the Candida tropicalis strain evaluated in their study (2.6μg IAA/ml). The literature on IAA production by yeasts reports a wide range of production levels, including 3–22μg IAA/ml11, 2–30μg IAA/ml14 and up to 600μg IAA/ml2. In addition to the potential influence of different detection techniques in the final yield, our findings support the claim that the level of IAA-like compound production is strain-specific5,7,13. Several IAA biosynthetic pathways have been proposed in a wide variety of microorganisms, and tryptophan is the most common precursor for IAA biosynthesis, though tryptophan-independent pathways also exist4. The tryptophan-dependent IAA biosynthesis pathways include two well-studied routes, named after their intermediate compounds: the indole-3-pyruvic acid pathway and the indole-3-acetamide pathway4. In the present study, we were able to detect by HPLC the presence of IAA in culture supernatant from S. eubayanus CRUB2014 and C. saitona CRUB1770. Nevertheless, the IAA peak (retention time 12.6min) was not detected in T. pullulans CRUB1772 cultures, which was the strain with the highest concentration of IAA-like compound determined by the colorimetric technique. The three yeast strains showed a peak at a retention time of 11.7min, and this peak had a significant area in T. pullulans CRUB1772 supernatant. Nassar et al.11 reported the production of the intermediate compound indole-3-pyruvic acid in the IAA biosynthetic pathway in W. saturnis and R. glutini. Our hypothesis is that the peak at a retention time of 11.7min might correspond to an intermediate compound in the IAA biosynthetic pathway that is accumulated in T. pullulans CRUB1772, instead of IAA. Furthermore, Glickman and Dessaux6 reported that the colorimetric technique using Salkowski detects indole-3-pyruvic acid and indole-3-acetamide as well as IAA. These intermediate compounds may be adding up to the concentration recorded with the colorimetric determination. This supports the hypothesis of the presence of intermediate compounds in the supernatant, possibly indole-3-pyruvic acid, and/or indole-3-acetamide. Therefore, positive results when using the colorimetric technique could include several IAA-related compounds. This is why we encourage the use of the statement “auxin-like compound production” or “IAA-like compound production” (when using IAA as standard for quantification) to describe the results obtained using colorimetric detection techniques to avoid ambiguous or misleading result interpretation.
Temperature is a major driver for microorganism metabolism, as evidenced by a lower concentration of IAA-like compounds detected in our work at 20°C for A. brasilense Az39 (whose growth temperature ranges from 20 to 38°C) than previously reported3. This suggests that the use of inoculants based on mesophilic PGPM such as A. brasilense Az39 in cold-temperate regions may not render the expected results. The three yeasts strains used in our study presented optimal growth temperatures under 25°C and endured freezing conditions in soil; therefore, the use of such psychrotolerant yeasts9 could be a more suitable tool in cold-temperate production environments. The intermediate compounds produced by the yeasts may not directly affect plant metabolism, but they may be used by other PGPM in the soil. Hence, it would be worth exploring the ability of T. pullulans CRUB1772 to enhance IAA biosynthetic pathways in A. brasilense Az39 and other PGPM by supplying intermediate compounds (moving forward the IAA biosynthetic pathway) at a low temperature, which may lead to a novel tool for sustainable agricultures in cold-temperate regions.
To conclude, the present manuscript confirmed the production of IAA by two of the studied Patagonian yeasts, along with other possible IAA-related yet unknown compounds using HPLC. Nowadays, HPLC is a separation and quantification technique adopted worldwide; nevertheless, its application in developing countries could somehow be limited due to financial restrictions. Additionally, the use of colorimetric techniques with Salkowski's reagent (a simple, fast and cheap routine assay) is still an invaluable tool for large-scale screening of auxin-like compound production, which allows the selection of potential plant growth promoting microorganisms (including yeasts). Here we present a novel HPLC protocol for the detection of IAA using minimal sample preparation, avoiding time- and money-consuming phytohormone extraction methods. This protocol could be used to confirm the presence of IAA in samples from large-scale screening using colorimetric techniques. To our knowledge, this is the first confirmed report of IAA production in cold-adapted yeasts from Patagonia, which also opens a novel line of research on the study of IAA biosynthetic pathways in yeasts in Argentina.
FundingThis work was supported by the “Universidad Nacional del Comahue-Centro Regional Universitario Bariloche” under Grant 04-B200; “Fondo para la Investigación Científica y Tecnológica (FONCYT)” under Grant PICT 2018-3441.
Conflict of interestThe authors declare that they have no conflicts of interest.