The detection of characteristics associated with plant growth promotion has been studied frequently on bacteria and some of these detection methods were also used on yeasts. Sometimes, these methods, designed for prokaryotes, were used with no prior analysis regarding growth and production on eukaryotes. The aim of the present study was to assess and select the best suitable media for the detection of auxin-like compound production and inorganic-P solubilization on yeast strains. Already published media and new formulations, as well as yeasts with different genetic backgrounds were used for the comparison. Media were selected based on the adequate growth of yeast strains and reliable recognition of the studied features either by an easy detection of the metabolite (color or halo production) or simple medium preparation (low number of reagent and regular autoclaving). We propose here the use of the new DEV medium with glucose and with tryptophan to identify auxin-like compounds producers; and CPM medium (a variation from Custer's Chalk Medium) as base medium to identify yeasts capable of inorganic-P solubilization.
La detección de características relacionadas con la promoción del crecimiento vegetal ha sido estudiada frecuentemente en bacterias; algunos de los métodos de detección también han sido usados en levaduras. En muchos casos, estos métodos se utilizaron en levaduras sin haber hecho análisis previos respecto de cómo funcionan para el crecimiento y la producción de compuestos por parte de estos eucariotas. El presente trabajo tuvo como objetivo evaluar y seleccionar los medios de cultivo adecuados para detectar solubilización de P inorgánico y producción de compuestos de tipo auxinas en levaduras. Para ello se utilizaron diferentes levaduras y se realizaron comparaciones empleando medios de cultivos conocidos y nuevas formulaciones adaptadas al crecimiento de las levaduras. Los medios de cultivo fueron seleccionados sobre la base del crecimiento adecuado de las levaduras y el reconocimiento fiable de los rasgos estudiados, ya sea por una determinación simple del metabolito (producción de color y halos) o por la sencillez en la preparación del medio de cultivo. Se propone aquí el uso del medio DEV adicionado con glucosa más triptófano para identificar levaduras productoras de compuestos de tipo auxinas y del medio CPM (una variación del medio Custer's con carbonato de calcio) como medio base para identificar levaduras capaces de solubilizar P inorgánico.
Physiological features associated with plant growth promotion commonly used on microorganism screening are production of auxin-like compounds, siderophores and polyamines, solubilization of inorganic-P, ACC-deaminase activity, and the ability of reducing pathogen growth.4 The study of plant growth promoting features in yeasts have drawn attention in recent years; however, it is a relatively unexplored subject. Published studies on yeasts have usually evaluated relevant features using media and methods developed for bacteria1,5,11,12. The selection of media for physiologic determination is not trivial, the use of inadequate medium could lead to overlook microorganisms with important biotechnological features. The present manuscript reports on the evaluation of different culture media mentioned in the literature and some new modifications, to propose reliable media for the detection of auxin-like compounds production and phosphate solubilization on yeast strains. Media evaluation was performed on four different yeasts including Basiodiomycota and Ascomycota strains: Tausonia pullulans CRUB 1772 and Lachancea nothofagi CRUB2011 were used for auxin-like compound production and inorganic-P solubilization; Candida saitoana CRUB 1770 was used for auxin-like compounds production and Saccharomyces eubayanus CRUB 2014 for inorganic-P solubilization. All yeasts are deposited at the Yeast Collection of Centro Regional Universitario Bariloche, Universidad Nacional del Comahue. T. pullulans and C. saitoana were previously described as potential plant growth promoting yeasts or PGPY9, as auxine-like compound producer and inorganic P solubilizers, respectively. L. nothofagi, a novel species from Patagonia, and S. eubayanus showed positive results for organic acid production8,10, a proxy for inorganic P-solubilization.
The most widely used technique for the detection of auxin-like compounds is based on colorimetric estimation of 3-indoleacetic acid (IAA) on culture supernatant6. Several IAA biosynthetic pathways, both tryptophan-dependent and independent, have been proposed3. Yeast studies used the same colorimetric estimation of IAA on different liquid media with or without tryptophan: glucose peptone broth11, YPD5,12,14 and DEV10. In order to analyze the appropriate medium for yeasts, two in vitro analyses were done. A first experiment, YPD (yeast extract 10g/l, soy peptone 20g/l, dextrose 20g/) and DEV (NaCl 5g/l, soy peptone 10g/l) were evaluated in their typical composition with or without tryptophan (0.1%, Trp). A second experiment was performed using a modified composition on both media, by adjusting glucose concentration (5g/l): adding glucose to DEV (DEV+glu) and reducing glucose concentration in YPD (YPD-glu). In both experiments, tubes containing 1.8ml of liquid medium were inoculated with 0.2ml of a cell suspension (NaCl 0.85% in water; DO600nm=0.3). Cultures were incubated for 7 days at 20°C, in triplicate. Determinations of culture growth were performed by measuring absorbance at 600nm. Detection of auxin-like compounds was performed using Salkowski's reagent (12g/l FeCl3, 7.9M H2SO4) and absorbance determination at 530nm6. Auxin-like compounds production was calculated using a reference curve with commercial IAA (Sigma), informed as IAA units (mM). Statistical analysis was performed using R software. The effect of media composition on yeast growth and auxin-like compound production was evaluated using the Kruskal–Wallis and Kruskal multiple comparison post hoc tests. All yeast cultures with positive growth were compared (10 levels for the independent variable). Auxin-like compound production results from the second experiment were Log10-transformed to fit the assumption for the statistical analysis. All yeast cultures with positive growth were compared (12 levels for the independent variable) The well-known PGPR bacterium Azospirillum brasilense (Az39) was used as positive control for the colorimetric technique; however, it was not included in the statistical analysis.
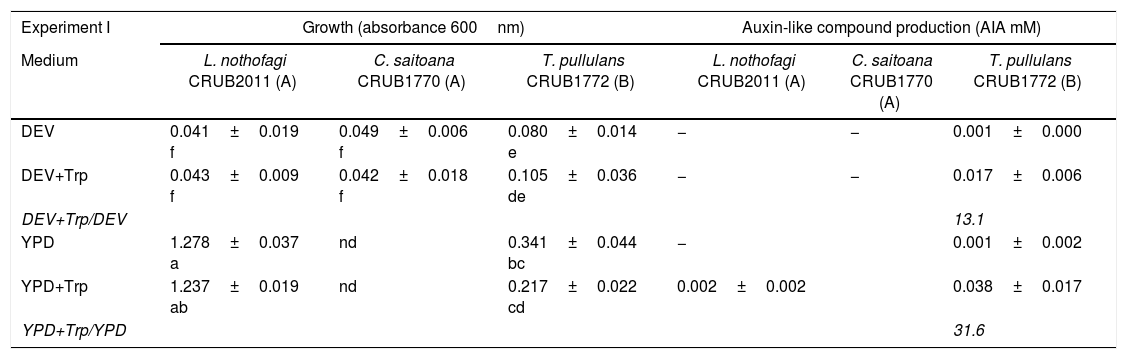
The results of yeast growth and auxin-like compound production obtained are shown in Table 1. In the first experiment, the growth of yeasts was different on the four media tested. Three yeasts showed very weak growth on DEV-based media. L. nothofagi and T. pullulans grew at a much higher level on YPD-based media than on DEV-based media, while C. saitoana showed no growth on YPD-based media. As a general feature, yeast growth using DEV as base medium was lower than YPD, probably due to the dextrose content. In this experiment, T. pullulans CRUB1772 was the only yeast that was able to produce auxin-like compounds on DEV and YPD-based media with tryptophan, while the other yeasts showed little or no production at all (Table 1). In the second experiment, both media were modified to contain the same final dextrose concentration (0.5%); as a result, the growth of all yeasts was enhanced on DEV+glu media and only C. saitoana and T. pullulans improved growth on YPD-glu media. The detection of auxin-like compounds was possible on all yeast cultures, while the production levels differed among yeasts. Auxin-like compound production was improved on media with tryptophan compared with media without tryptophan, which indicates that tryptophan is needed to produce these compounds. L. nothofagi seems to be a poor producer of auxin-like compounds. C. saitoana and T. pullulans auxin-like compound production was highest on DEV+glu+Trp media. These yeasts seem to produce auxin-like compounds even at relatively low levels of growth: DO600nm was 0.271 and 0.475 for C. saitoana and T. pullulans, respectively. T. pullulans seems to be a good producer of auxin-like compounds on any base media when tryptophan is added (Table 1) compared with the other two yeasts. The auxin-like compound production level of yeasts on any media was lower than the control (bacterial strain Az39). Nevertheless, the levels of production were higher on DEV+glu+Trp than YPD-glu+Trp, even when low growth of the bacteria was registered (Table 1). The negative result of auxin-like compound production on DEV media from the first experiment could be attributed to the low growth of yeasts. We addressed this fact in the second experiment by modifying the dextrose concentration. Autoclaved YPD sometimes exhibited a darker color which varied depending on the medium batch and could interfere with determinations. We reduced color variation in the second experiment by modifying the dextrose content. We propose the use of DEV+glu+Trp medium for auxin-like compound production screening on yeasts due to higher production, simple composition and lack of color change during the autoclave procedure.
Growth and auxin-like compounds production of yeasts on different culture media. Variables are expressed as mean±standard deviation.
| Experiment I | Growth (absorbance 600nm) | Auxin-like compound production (AIA mM) | ||||
|---|---|---|---|---|---|---|
| Medium | L. nothofagi CRUB2011 (A) | C. saitoana CRUB1770 (A) | T. pullulans CRUB1772 (B) | L. nothofagi CRUB2011 (A) | C. saitoana CRUB1770 (A) | T. pullulans CRUB1772 (B) |
| DEV | 0.041±0.019 f | 0.049±0.006 f | 0.080±0.014 e | − | − | 0.001±0.000 |
| DEV+Trp | 0.043±0.009 f | 0.042±0.018 f | 0.105±0.036 de | − | − | 0.017±0.006 |
| DEV+Trp/DEV | 13.1 | |||||
| YPD | 1.278±0.037 a | nd | 0.341±0.044 bc | − | 0.001±0.002 | |
| YPD+Trp | 1.237±0.019 ab | nd | 0.217±0.022 cd | 0.002±0.002 | 0.038±0.017 | |
| YPD+Trp/YPD | 31.6 | |||||
| Experiment II | Growth (absorbance 600nm) | Auxin-like compound production (AIA mM) | ||||||
|---|---|---|---|---|---|---|---|---|
| Medium | L. nothofagi CRUB2011 (A) | C. saitoana CRUB1770 (A) | T. pullulans CRUB 1772 (B) | A. brasilense Az39 (control) | L. nothofagi CRUB2011 (A) | C. saitoana CRUB1770 (A) | T. pullulans CRUB 1772 (B) | A. brasilense Az39 (control) |
| DEV+glu | 0.882±0.026 a | 0.820±0.120 ab | 0.501±0.051 c | 0.468±0.014 | 0.003±0.002 de | 0.003±0.001 e | 0.001±0.000 f | 0.009±0.001 |
| DEV+glu+Trp | 0.788±0.009 b | 0.271±0.060 d | 0.475±0.094 c | 0.456±0.066 | 0.007±0.001 cd | 0.027±0.007 ab | 0.036±0.008 a | 0.126±0.015 |
| DEV+glu+Trp/DEV+glu | 1.2 | 9.0 | 36.0 | 14.7 | ||||
| YPD-glu | 0.880±0.048 a | 0.291±0.115 d | 0.758±0.087 b | 0.805±0.150 | 0.001±0.000 f | 0.004±0.001 e | 0.004±0.001 e | 0.002±0.001 |
| YPD-glu+Trp | 0.834±0.022 ab | 0.206±0.023 d | 0.482±0.045 c | 0.942±0.103 | 0.004±0.001 cd | 0.010±0.003 bc | 0.030±0.004 ab | 0.082±0.024 |
| YPD+glu+Trp/YPD+glu | 4.0 | 2.5 | 7.5 | 43.0 | ||||
(A): Ascomycota; (B): Basidiomycota; −: negative; nd: not detected. Letters indicate different homogenous groups calculated by the KW multiple comparison test among yeast cultures (10 in experiment I and 12 in experiment II), for growth and auxin-like compounds individually. Bold characters correspond to highest level of production.
Inorganic P-solubilization by microorganisms is mostly attributed to the production of organic acids. The released P results from either lowering pH, chelation of cations or complexation of metal bonds to P, or from competition for P-absorption sites in soil13. The most widely used method for the detection of inorganic P-solubilization rely on the formation of a clear halo surrounding the colonies growing on the solid medium with tricalcium phosphate [Ca3(PO4)2]. Some researchers have resisted the use of tricalcium phosphate as sole selection factor and proposed the simultaneous use of diverse P-insoluble compounds2. The most commonly used medium to test phosphate solubilization ability on yeasts is Pikovskaya1,5,12 and YPD in previous works9. In the present study four media were tested: Pikovskaya [PKV: MgSO4·7H2O 0.15g/l, MnSO4·4H2O (10% v/v) 0.15ml/l; (NH4)2SO4 0.75g/l, KCl 0.3g/l, yeast extract 0.75g/l glucose 15g/l and agar 15g/l]; YPD (yeast extract 10g/l, soy peptone 20g/l, dextrose 20g/l); CPM medium (yeast extract 5g/l, glucose 50g/l and agar 15g/l) and modified CPM with lower glucose content (20g/l) called CPM-glu; 0.5% of HCaPO4 was added to all media. Custer's Phosphate Medium (CPM) is based on Custer's Chalk Medium, used for the determination of organic acid production in the physiological identification of yeasts using CaCO37; here chalk was exchanged for HCaPO4. The solubility of HCaPO4 in water (0.02g/100ml) is higher than in Ca3(PO4)2 (0.002g/100ml) or CaCO3 (0.0013g/ml), used in the Custer's chalk medium. The use of HCaPO4 allowed us to search for a medium to be used as a base for testing different P-insoluble compounds in short term cultures. Triplicate plates were inoculated with a 10μl drop of a cell suspension (NaCl 0.85% in water; DO600nm=0.3), incubated for 7 days at 20°C and examined daily. Positive results were observed as clear halos around the yeast colony. Semi-quantitative determination was performed comparing colony and halo diameter. The yield of HCaPO4 solubilization (%YP) was determined as: [(halo diameter−colony diameter)/colony diameter]×100. Statistical analysis was performed using R-software. All yeast cultures with positive halo formation were compared. The effect of media composition on colony diameter (yeast growth) and solubilization halo diameter was evaluated using the Kruskal–Wallis test and the Kruskal multiple comparison post hoc test (nine levels). Solubilization yield results were square-root transformed to fit a two-way ANOVA (independent factors used were yeast strain and medium) assumption, followed by the post hoc Tukey test. Pseudomonas protegens Cha0 was used as positive control for the detection; however, it was not included in the statistical analysis.
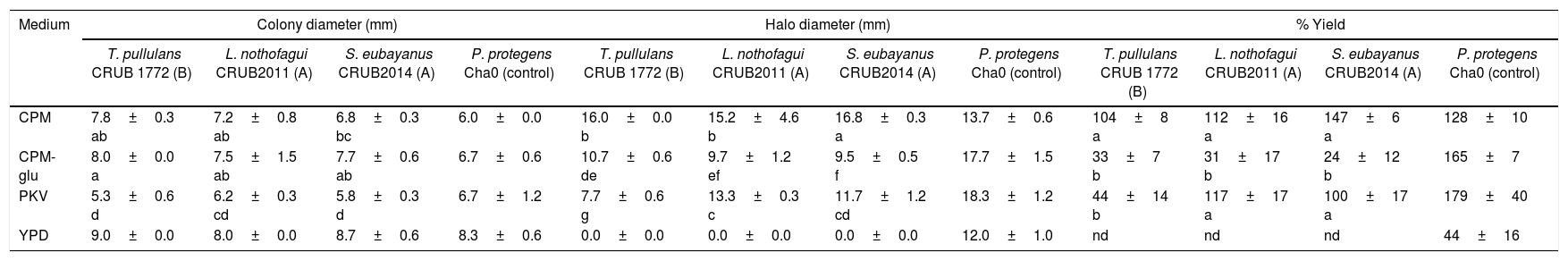
The results for colony diameter (yeast growth), halo diameter and percentage yield obtained are shown in Table 2. All yeasts and the bacterial control were able to grow on the four media tested. Yeast colony diameters on PKV plates were the smallest compared to the other 3 media, which showed low growth. After 72h incubation, halos formed by yeasts were observed on CPM, CPM-glu and PKV media but they were absent on YPD medium. Halos produced by yeasts on the CPM-glu medium were difficult to measure due to their small diameter and diffuse borders. CPM and PKV media showed visible solubilization halos and yield was similar on both media for ascomycetous yeasts L. nothofagi and S. eubayanus. For these yeasts, the CPM medium allowed a better halo identification (color and borders) than PKV at 72h. T. pullulans’ halos on PKV medium were similar to those formed on CPM. Bacterial control strains P. protegens Cha0 showed the best results for solubilization yield and halo diameter on PKV (most commonly used media for bacterial screenings). CPM medium seems to be a good alternative when performing screening studies that include yeasts (Basidiomycota and Ascomycota) and bacteria due to a high level of yield for all the strains tested. Solubilization yield increased with time; however, the risk of plate contamination also increased. When using Ca3(PO4)2, halo development takes one or two weeks due to low solubility (unpublished results). The results presented here allowed us to select the CPM medium for phosphate solubilization screening on yeasts in short periods of time. If other P-insoluble compounds were used, a longer period of incubation should be considered.
Growth and phosphate solubilization on yeast cultures after 72h of incubation. Variables are expressed as mean±standard deviation.
| Medium | Colony diameter (mm) | Halo diameter (mm) | % Yield | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. pullulans CRUB 1772 (B) | L. nothofagui CRUB2011 (A) | S. eubayanus CRUB2014 (A) | P. protegens Cha0 (control) | T. pullulans CRUB 1772 (B) | L. nothofagui CRUB2011 (A) | S. eubayanus CRUB2014 (A) | P. protegens Cha0 (control) | T. pullulans CRUB 1772 (B) | L. nothofagui CRUB2011 (A) | S. eubayanus CRUB2014 (A) | P. protegens Cha0 (control) | |
| CPM | 7.8±0.3 ab | 7.2±0.8 ab | 6.8±0.3 bc | 6.0±0.0 | 16.0±0.0 b | 15.2±4.6 b | 16.8±0.3 a | 13.7±0.6 | 104±8 a | 112±16 a | 147±6 a | 128±10 |
| CPM-glu | 8.0±0.0 a | 7.5±1.5 ab | 7.7±0.6 ab | 6.7±0.6 | 10.7±0.6 de | 9.7±1.2 ef | 9.5±0.5 f | 17.7±1.5 | 33±7 b | 31±17 b | 24±12 b | 165±7 |
| PKV | 5.3±0.6 d | 6.2±0.3 cd | 5.8±0.3 d | 6.7±1.2 | 7.7±0.6 g | 13.3±0.3 c | 11.7±1.2 cd | 18.3±1.2 | 44±14 b | 117±17 a | 100±17 a | 179±40 |
| YPD | 9.0±0.0 | 8.0±0.0 | 8.7±0.6 | 8.3±0.6 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 12.0±1.0 | nd | nd | nd | 44±16 |
nd: not detected. (A): Ascomycota; (B): Basidiomycota. Letters indicate different homogenous groups calculated by the KW multiple comparison test among nine yeast cultures. Capital letters indicate different homogenous groups calculated by the Tukey test.
Several media have been used for the detection of plant growth promoting (PGP) features on yeasts and most of them were initially developed for bacterial studies. The present report searches for the best suitable media for detection of auxin-like compounds production and inorganic-P solubilization on yeasts. Already published and newly proposed media, as well as yeasts with different genetic backgrounds were used for the comparison. Media were selected based on the adequate growth of yeasts and reliable recognition of the studied features either by an easy detection of the metabolite (color or halo production) or simple medium preparation (low number of reagents and regular autoclaving). We propose the use of the DEV+glu medium with tryptophan to identify auxin-like compound producers; and the CPM medium (a variation of the Custer's Chalk Medium) as base-medium to identify yeasts capable of inorganic-P solubilization.
Conflict of interestThe authors declare that they have no conflicts of interest.
FundingThis work was supported by FONCYT2014-PICT1839 and PFIP-ESPRO2013 (Disp. 007/13) from the MINCyT Argentina.