Fungal hydrolysis of ellagitannins produces hexahydroxydiphenic acid, which is considered an intermediate molecule in ellagic acid release. Ellagic acid has important and desirable beneficial health properties. The aim of this work was to identify the effect of different sources of ellagitannins on the efficiency of ellagic acid release by Aspergillus niger. Three strains of A. niger (GH1, PSH and HT4) were assessed for ellagic acid release from different polyphenol sources: cranberry, creosote bush, and pomegranate used as substrate. Polyurethane foam was used as support for solid-state culture in column reactors. Ellagitannase activity was measured for each of the treatments. Ellagic acid was quantified by high performance liquid chromatography. When pomegranate polyphenols were used, a maximum value of ellagic acid (350.21mg/g) was reached with A. niger HT4 in solid-state culture. The highest amount of ellagitannase (5176.81U/l) was obtained at 8h of culture when cranberry polyphenols and strain A. niger PSH were used. Results demonstrated the effect of different polyphenol sources and A. niger strains on ellagic acid release. It was observed that the best source for releasing ellagic acid was pomegranate polyphenols and A. niger HT4 strain, which has the ability to degrade these compounds for obtaining a potent bioactive molecule such as ellagic acid.
La hidrólisis fúngica de los elagitaninos produce ácido hexahidroxidifénico, considerado como una molécula intermedia en la liberación de ácido elágico. El ácido elágico tiene importantes y deseables propiedades benéficas para la salud humana. El objetivo de este trabajo fue identificar el efecto de la fuente de elagitaninos sobre la eficiente liberación de ácido elágico por Aspergillus niger. La liberación de ácido elágico se realizó con tres cepas de A. niger (GH1, PSH y HT4) en presencia de diferentes fuentes de polifenoles (arándano, gobernadora y granada), usadas como sustrato. Se empleó espuma de poliuretano como soporte para el cultivo en estado sólido en reactores en columna. Se midió la actividad elagitanasa a cada uno de los tratamientos. El ácido elágico liberado se cuantificó por cromatografía líquida de alta resolución. Cuando se utilizaron los polifenoles de granada, se alcanzó un valor máximo de 350,21mg/g de ácido elágico con A. niger HT4 en cultivo en estado sólido. La mayor actividad elagitanasa (5176.81 U/l) se obtuvo a 8 h de cultivo cuando se usaron los polifenoles de arándano como sustrato y A. niger PSH. Los resultados demostraron el efecto que tiene la fuente de polifenoles y la cepa de A. niger en la liberación de ácido elágico. Se observó que la mejor fuente para la liberación de ácido elágico fueron los polifenoles de granada y que la cepa A. niger HT4 posee la habilidad de degradar estos compuestos para la obtención de potentes moléculas bioactivas, como el ácido elágico.
Polyphenols are a wide range of compounds considered to be secondary metabolites of different plant parts: leaves, stems, flowers, fruits, seeds, and others2. Polyphenols are divided into three main groups: complex tannins, condensed tannins and hydrolyzable tannins17. Ellagitannins are a class of hydrolyzable tannins. These compounds are essentially formed by a hexahydroxydiphenic acid (HHDP) group linked by an ester bond to a glucose8. There are over 500 different structures of ellagitannins reported in the literature13. Ellagitannins are mainly obtained from the bark and trunks of trees such as oak (Quercus spp.)18 and eucalyptus (Eucalyptus spp.)19. Ellagitannins are abundant in some red berries24, plants of the Mexican desert6 and pomegranate fruit22. Ellagitannins have various beneficial health properties. It has been shown that the ellagitannins present in pomegranate juice can induce apoptosis by activation of the leukemic cell cycle11. Punicalagin is an ellagitannin from the pomegranate peel, which has been linked to apoptosis in HT-29 colon cancer cells23. Ellagitannins present in leaf extracts, flower extracts, peel, juice and seeds of pomegranate have beneficial effects on the control of obesity, diabetes, cardiovascular disease, hypertension and hypercholesterolemia5. Moreover, these polyphenols in pomegranate leaves, obtained by macroporous resin column chromatography have a high antioxidant activity29. Ellagitannins can be hydrolyzed by chemical or enzymatic methods to release the HHDP group15; this group undergoes a spontaneous molecular arrangement forming a new molecule called ellagic acid (EA)3. EA is a secondary metabolite of high industrial interest due to various beneficial effect on human health such as antiviral16, anticarcinogenic20, antioxidant26, and anti-inflammatory activities10. Industrially, EA is obtained with the use of chemicals, involving high production costs and recovery as well as environmental damage. Therefore, it is necessary to develop methods for the biotechnological production of secondary metabolites. There are few studies about obtaining EA by bioprocessing agroindustrial residues such as pomegranate peel and microorganisms that are capable of degrading the ellagitannin compounds present in the waste by solid-state culture (SSC) to generate EA25. The aim of this work was to evaluate the effect of different polyphenol sources: cranberry (CP), creosote bush (CBP) and pomegranate (PP) on the hydrolytic efficiency of three different xerophilic Aspergillus niger strains, denominated A-GH1, A-PSH and A-HT4, to obtain EA by SSC.
Materials and methodsPolyphenol extraction and purificationCranberries (Vaccinium macrocarpon Ait.) were originally from Wisconsin, USA, and were received in vacuum packed bags (350g) in the month of January of the year 2012. Pomegranates (Punica granatum L. var. Wonderful) were originally from California, USA, and were received in cardboard boxes (25kg), in the same session and year. These fruits were purchased at a supermarket in Saltillo, Coahuila, Mexico. Creosote bush plants (Larrea tridentate Sesse & Mocino ex DC. Coville) were manually collected in Federal Highway 57 along 15km of the Saltillo-Monclova stretch, in Coahuila State, Mexico in the second semester of the year 2011. Pomegranate peel, cranberry and creosote bush leaves were dehydrated at 60°C for 48h, then pulverized in a grinder (PULVEX® Mini 100) to obtain a particle size of 600μm and taken to constant weight25. Polyphenols were extracted according to the methodology proposed by Ascacio-Valdés et al6. Substrate was hydrated at 1:5 ratio (100g substrate/500ml distilled water) and incubated at 60°C for 30min. The substrate mass was filtered through muslin cloth and the extract obtained was centrifuged at 3000rpm for 15min. The supernatant was purified by chromatography (Amberlite XAD-resin 16®). The extracts were eluted with distilled water (dH2O) to remove soluble compounds. The polyphenolic compound-rich fraction was eluted with 96% ethanol. Finally, the ethanol was evaporated in a heating oven (NAPCO® Model 322) at 60°C for 24h. The CP CBB and PP were obtained as a fine powder.
Support preparationThe use of polyurethane foam (PUF) as inert support for polyphenol biodegradation was assessed. The PUF was cut and ground to a particle size of 0.85mm, then washed (3×) with hot water (80–90°C) for 10min, filtered to eliminate and dried until constant weight21.
Microorganisms and culture mediaA. niger GH1, A. niger PSH and A. niger HT4 strains were supplied by DIA-UAdeC (Departamento de Investigación en Alimentos, Universidad Autónoma de Coahuila). These strains have the ability to degrade substrates rich in polyphenols3,12,22. The strains were activated in potato dextrose agar (PDA-Bioxon), and incubated at 30°C for 5 days. Spores were harvested with 0.01% Tween-80 and counted in a Neubauer® chamber. Pontecorvo culture medium having the following composition (g/l): NaNO3 6.0, KH2PO4 1.52, KCl 0.52 MgSO4·7H2O 0.52, ZnSO4 0.001, FeCl 0.85 and 1ml trace metals was used. The trace metal solution contained (mg/l) Na2B4O7·10H2O 10, MnCl2·4H2O 50, Na2MoO4·2H2O 50 and CuSO4·5H2O 250. The carbon source (30g/l) was supplemented with either CP, CBP or PP7 and the initial culture medium pH was adjusted to 6.5 with 1M NaOH. An inoculum size of 2×107 spores per g of culture material was used.
SSC conditionsThe SSC was prepared as follows: 0.5g of PUF was homogenized with 1.16ml of culture medium (70% humidity) and packed in columns (0.5cm×5cm) at a packing density of 0.08g/cm3. The solid-state culture was performed for 32h at 30°C, with sampling every 8h (two columns by treatment)9. The enzymatic extract of the culture was obtained by adding 1.16ml of 50mM citrate buffer (pH 5) within each column, the mass culture was mixed and the extract was obtained by manual pressing. To recover the EA, an ethanol–formic acid (1.16ml of 0.01%) solution was added25 and the extracts obtained were filtered (0.45μm) and frozen until analysis.
Ellagitannase activity assayEllagitannase activity was carried out according to the methodology suggested by De la Cruz et al12. Ellagitannins 1mg/ml in 50mM citrate buffers, pH 5, were used as enzyme substrate. A substrate control (1ml ellagitannins and 50μl of 50mM citrate buffers, pH 5), enzyme control (1ml of 50mM citrate buffers, pH 5 and 50μl of enzymatic extract) and the reaction mixture (1ml of ellagitannins and 50μl of enzymatic extract) were prepared. The reaction was done in a water bath for 10min at 60°C. The reaction was stopped by adding 1050μl of absolute ethanol. Then, samples were sonicated for 25min, filtered through 0.45μm nylon membrane and collected in vials. One ellagitannase enzymatic unit was defined as the enzyme amount needed to release 1μmol of EA per min, under the conditions described.
HPLC analysisQuantification of EA was performed on a Varian ProStar model 2301 HPLC. A column Pursuit XRs 5um C18 (150mm×46mm) and, (A) methanol (washing phase), (B) acetonitrile and (C) acetic acid 3% (mobile phase). The flow rate was 1ml/min, at a column temperature of 30°C. Standard curve of EA (≥95%, Sigma–Aldrich®) from 0 to 500μg/ml was prepared7.
Data analysisThe effect of the different polyphenol sources and strains on EA accumulation was assessed under a 3×3 factorial design with two replicates. Data were analyzed by ANOVA using SAS 9.0 and means were compared by Tukey's multiple range procedure (p≤0.05) when needed.
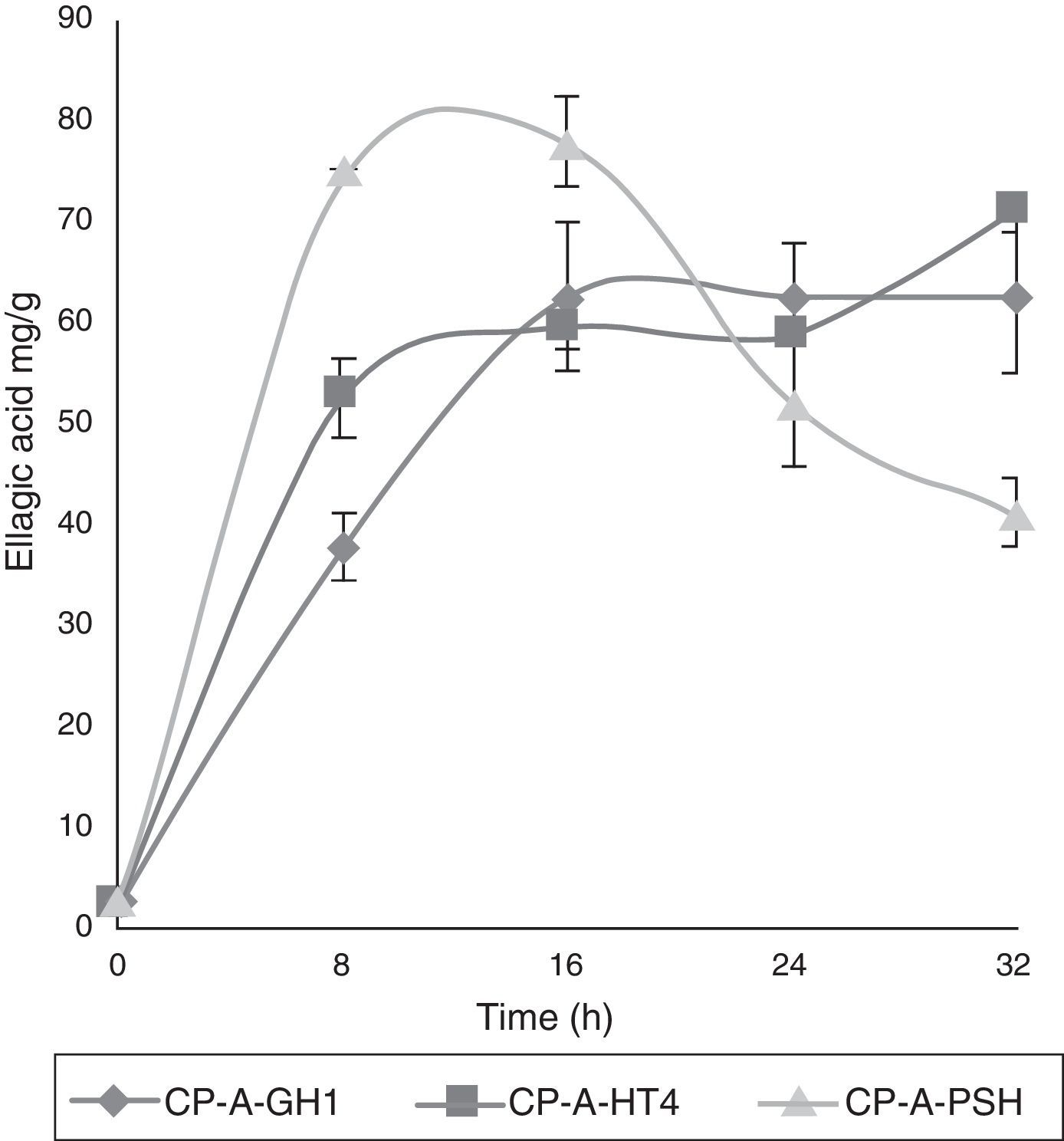
ResultsKinetics of EA accumulationCulture kinetics of A-GH1, A-PSH and A-HT4 strains and three culture media enriched with CP, CBP and PP were studied. The kinetic profile when using CP as polyphenolic substrate (Fig. 1) showed the highest EA accumulation (74.56mg/g) with the A-PSH strain at 8h of culture followed by the A-HT4 strain (69.55mg/g) at 32h of culture and A-GH1 (64.35mg/g) at 32h of culture. The A-HT4 strain produced 13.69% more EA than the A-GH1 strain; however the former strain also required a 4-fold increase in the culture time required by the A-GH1 strain. Therefore, in terms of process time, A-GH1 resulted in higher EA accumulation than A-HT4. Fig. 2 shows the EA accumulation profile when using CBP. Total EA accumulation by the different strains was 182.92, 158.54 and 125.97mg/g using A-GH1, A-HT4 and A-PSH strains respectively; however, A-HT4 reached 158.54mg/g in an 8h culture while A-GH1 reached in 32h culture. Once again, a lower EA accumulation value (158.54mg/g) could be observed in one of the strains tested (A-HT4) but a culture time 4 times lower than in strain A-GH1 that showed the highest EA accumulation (182.92mg/g). In contrast, when using PP as substrate, EA accumulation was 163.03, 350.21 and 200.33mg/g for A-GH1 (8h culture), A-HT4 (16h of culture) and A-PSH (16h culture) respectively; the highest EA accumulation occurred at 16h of culture when using A-HT4 and no EA increase was observed at any other time (Fig. 3).
Ellagitannase activity reached maximum value of 5176.81U/l when using CP as carbon source and A-PSH at 8h of SSC (Fig. 4). Other strains reached maximum values in the first 8h and then these values were kept constant. Table 1 shows effect of substrates, strains and interaction on ellagitannase activity and EA productivity. EA productivity was defined as the ratio among the maximum accumulation of EA per culture time. In Fig. 5, the values of EA productivity are displayed at a time of 16h, which corresponds to the time of maximal EA accumulation obtained. In Table 2, the individual effect of each variable, validated by a Tukey test on ellagitannase activity and EA productivity is shown. Then the highest productivity (21.89mg/g/h), was obtained when using the Aspergillus strain coded as A-HT4 and CP as source. It was observed that when A-HT4 and CP were used, a maximum productivity of 21.89mg/g/h was reached.
Analysis of variance for EA productivity and ellagitannase activity
| Responses | Source of variation | dfa | MSb | p-Value | F-test |
|---|---|---|---|---|---|
| EA productivity | Replicate | 1 | 0.00 | 0.98 | 0.00 |
| Sc | 2 | 1252.51 | <.0001 | 858.68 | |
| Xd | 2 | 487.89 | <.0001 | 334.43 | |
| S*X | 4 | 382.99 | <.0001 | 262.52 | |
| R2=0.997 | |||||
| Ellagitannase activity | Replicate | 1 | 17034.93 | 0.26 | 1.50 |
| Sc | 2 | 43399.04 | 0.07 | 3.82 | |
| Xd | 2 | 230928.25 | <.0001 | 20.32 | |
| S*X | 4 | 112108.11 | <.0001 | 9.86 | |
| R2=0.917 | |||||
S*X=combined effect of substrate and strains.
Substrate and strain effect on EA productivity and ellagitannase activity
| Substrate | EA productivity (mg/g/h) | Ellagitannase activity (U/l) |
|---|---|---|
| PP | 40.59 a | 2721.38 a |
| PCB | 22.06 b | 2671.36 a |
| PC | 12.13 c | 2837.17 a |
| Strains | ||
| A-HT4 | 35.11 a | 2733.28 b |
| A-PSH | 21.76 b | 2944.31 a |
| A-GH1 | 17.92 c | 2552.33 c |
Means with the same letter, in the same column, are not significantly different according to the Tukey's multiple range test (p<0.05).
The A-PSH strain has been reported to have the ability to produce EA by hydrolysis of the pomegranate peel by SSC22. Moreover, this strain also showed the ability to degrade hydrolyzable tannins from extracts of creosote bush (L. tridentata) and tarbush (Flourensia cernua) releasing EA and gallic acid by SSC28. Furthermore, the A-GH1 strain has been reported for the hydrolysis of polyphenolic compounds from pomegranate peel, reaching 17mg/g of EA at 32h by SSC4. Vattem and Shetty (2003) reported the use of cranberry pomace and Lentinus edodes strain for EA production reaching a maximum accumulation of 350μg/g of EA by dried cranberry pomace and they attributed the EA accumulation to the β-glucosidase enzyme27. In this work, when A-HT4 strain and PP are used, a maximum of 350.21mg/g of EA at 16h is reached. It has also been reported that the pomegranate peel is an excellent carbon source for EA production, using an A. niger strain1. Recent studies mention that there is a possible set of enzymes that are responsible for the release of the HHDP group and formation consequence of EA7.
The values of ellagitannase activity obtained in this study are 166-fold higher than those reported by Aguilera-Carbó et al4. These authors reported values of 44.5U/l using pomegranate peel as carbon source and A-GH1 at 48h of culture time. Similar studies obtained maximum values of ellagitannase activity (2189.94U/l) at 30h of SSC using PP as carbon source and several fungal strains (A. niger PSH, A. niger GH1, A. niger HT4 and A. niger HC2)12. Several enzymes responsible for ellagitannin hydrolysis have been reported; however, the authors found that only ellagitannase, clearly associated with EA accumulation on SSC, reached maximum values (200.04U/l) at 12h of SSC using PP and A. niger GH1 strain7. The authors reported that EA production is higher using partially purified polyphenols, since these molecules have a glucose core, making them more susceptible to attack by microbial enzymes14. There are a few studies about the use of polyphenols from plants, barks or fruits SSC for obtaining secondary metabolites. The productivity obtained with CP and the A-HT4 strain is higher compared with that in previous reports using valonia tannins as a carbon source, reaching values of 0.92U/l/h11. The A-GH1 strain has been associated with the release of EA from creosote bush extracts by SSC, reaching a productivity of 1.22U/l/h4.
In conclusion, it was possible to obtain EA from rich substrates in polyphenolic compounds using A. niger strains in SSC. A-HT4 reached a maximum EA value (350.21mg/g of PP at SSC). When CP and the A-PSH strain was used, high values of ellagitannase activity (5176.81U/l) were obtained at 8h of SSC. The EA release mechanism is unknown due to EA complexity; however ellagitannase is one of the enzymes that may be responsible for the hydrolysis of the ester link in the HHDP group to generate EA.
Conflict of interestThe authors declare that they have no conflicts of interest.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
The authors would like to thank the Mexican Council of Science and Technology (CONACyT) for the scholarship granted for their postgraduate studies in the program of Food Science and Technology of the Autonomous University of Coahuila, Saltillo, México.