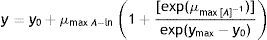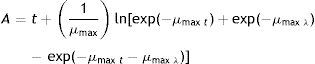Sorghum, which is consumed in Tunisia as human food, suffers from severe colonization by several toxigenic fungi and contamination by mycotoxins. The Tunisian climate is characterized by high temperature and humidity that stimulates mold proliferation and mycotoxin accumulation in foodstuffs. This study investigated the effects of temperature (15, 25 and 37°C), water activity (aw, between 0.85 and 0.99) and incubation time (7, 14, 21 and 28d) on fungal growth and aflatoxin B1 (AFB1) production by three Aspergillus flavus isolates (8, 10 and 14) inoculated on sorghum grains. The Baranyi model was applied to identify the limits of growth and mycotoxin production. Maximum diameter growth rates were observed at 0.99aw at 37°C for two of the isolates. The minimum aw needed for mycelial growth was 0.91 at 25 and 37°C. At 15°C, only isolate 8 grew at 0.99aw. Aflatoxin B1 accumulation could be avoided by storing sorghum at low water activity levels (≤0.91aw). Aflatoxin production was not observed at 15°C. This is the first work on the effects of water activity and temperature on A. flavus growth and AFB1 production by A. flavus isolates on sorghum grains.
El sorgo, que se consume en Túnez como alimento humano, puede sufrir la colonización severa de varios hongos toxicogénicos, con la consiguiente bioacumulación de micotoxinas. Además, el clima de Túnez, caracterizado por las altas temperaturas y humedad, estimula el crecimiento fúngico y la acumulación de micotoxinas en los productos alimenticios. Este estudio investigó los efectos de la temperatura (15, 25 y 37°C), la actividad de agua (aw) (entre 0,85 y 0,99) y el tiempo de incubación (7, 14, 21 y 28 días) sobre el crecimiento y la producción de aflatoxina B1 (AFB1) de 3 aislados de Aspergillus flavus (designados como 8, 10 y 14) que se inocularon sobre granos de sorgo. El modelo Baranyi se aplicó para identificar los límites del crecimiento y la producción de micotoxinas. Las tasas máximas de crecimiento para 2 de los aislados se observaron en la combinación 0,99 aw y 37°C. La aw mínima necesaria para el crecimiento del micelio fue de 0,91 a 25°C y 37°C. A 15°C, solo el aislado 8 creció a 0,99 aw, pero fue incapaz de producir la aflatoxina B1. Es posible evitar la acumulación de aflatoxina B1 en el sorgo almacenándolo a baja actividad de agua (≤ 0,91 aw). Este es el primer trabajo que ha estudiado el efecto de la actividad del agua y la temperatura sobre el crecimiento de aislados de A. flavus y su producción de aflatoxina B1 en granos de sorgo.
Aflatoxins are a group of toxic chemical compounds that are characterized by their immunotoxic, mutagenic and carcinogenic effects17. Aflatoxins are classified as group I carcinogens by the International Agency for Research on Cancer38. Among them, aflatoxin B1 (AFB1) is the most frequent and most potent toxin. A. flavus and to a lesser extent Aspergillus parasiticus are among the most widely studied fungal species, as a result of their ability to produce aflatoxins and their potential to persist pre- and post-harvest as a pathogen and saprophyte in the food supply.
The growth of molds and the accumulation of mycotoxins in food and feedstuff are influenced by multiple variables, such as water activity (aw), temperature, pH, atmosphere composition, substrate, interaction among species, and time3,37. Generally, relative humidity and temperature are considered to be the most critical factors during drying and storage14. Various physical and chemical methods have been recommended to reduce mycotoxins, but only a few have been accepted for practical use. Thus, the prevention of fungal growth and mycotoxin production represent key steps in risk management. Many kinetic models have been developed and used to model the growth of toxigenic molds in various food substrates. The effect of biotic and abiotic factors on growth and aflatoxin production have been widely studied using mathematical modeling4,6,14,16,18,20,28,39; however, no studies exist concerning their growth on sorghum seeds.
Sorghum (Sorghum bicolor (L.) Moench) is a drought-resistant crop and an important food resource in terms of nutritional and economic values, especially in semi-arid environments7. Sorghum grains can be colonized by several fungal genera during the panicle and grain development stages21,22. Several species of Aspergillus, Alternaria, Fusarium, Cladosporium, Curvularia and Penicillium are among the most prevalent grain mold pathogens in sorghum34. Moreover, a number of fungal isolates from this cereal primarily belonging to the genera Fusarium (F. verticillioides, F. graminearum, F. equiseti), Alternaria (A. alternata), Aspergillus (A. flavus), and Penicillium (P. funiculosum) have been reported to produce mycotoxins27,30,36.
The Tunisian climate is characterized by high temperature and humidity levels that seem to stimulate toxigenic mold growth and mycotoxin production. The Tunisian population consumes large amounts of cereals and cereal-based products, such as wheat, barley and sorghum. The occurrence of aflatoxins in commercialized sorghum in Tunisia was reported for the first time in 19772, and several studies confirmed aflatoxin contamination in Tunisian sorghum1,8,9,10,19. According to Oueslati et al.10, AFB1-contaminated cereals represent a real problem for Tunisian consumers. Therefore, it is important to develop prevention and control strategies to minimize aflatoxin contamination in sorghum.
The objectives of the present work were to determine: (i) the effects of the interactions of water activity, temperature and incubation time on fungal growth and aflatoxin B1 production by Tunisian Aspergillus flavus isolates cultured on sorghum seeds and (ii) to identify the aw and temperature limiting conditions for aflatoxin B1 production. The Baranyi model2 was applied to predict the growth of aflatoxigenic isolates of A. flavus on sorghum seeds as a function of water activity and temperature. Elucidating the ecology and physiology of A. flavus will provide tools to manage AFB1 in sorghum.
Materials and methodsFungal isolatesThree isolates of A. flavus (8, 10 and 14) were used in this study. The three isolates were obtained from sorghum samples collected from the retail market of the Sahel region in Tunisia in 2011 and were kept in the Food Technology department collection of the University of Lleida. All isolates were previously found to produce AFB1 when cultivated in Czapeck Yeast Extract Agar (CYA) and sorghum seeds. The identification of the A. flavus isolates was done microscopically29. Molecular characterization of the isolates was also performed by PCR amplification using the primer pair AfAflT-F and AfAflT-R35. Two DNA extracts of A. flavus [CECT (Spanish Type Culture Collection) 2695 and A. parasiticus CECT 2681] were used as references.
Experimental design and statistical analysisA full factorial design of three temperatures (15, 25 and 37°C)×six water activities (aw) (0.85, 0.88, 0.91, 0.94, 0.97 and 0.99)×three isolates (8, 10 and 14) was used. Colony diameters and AFB1 production after 7, 14, 21 and 28d were the dependent variables. Three replicates were included for each treatment. The effects of temperature and aw on mycelial growth and AFB1 production after 7, 14, 21 and 28d were statistically analyzed by analysis of variance (ANOVA) using Statgraphics Plus 5.1 (Manugistics, Inc., MD, USA).
Preparation of sorghumThe study was performed in vitro on sorghum grains. To determine the growth and aflatoxin patterns on sorghum grains, different amounts of water (calculated from a moisture absorption curve for the sorghum batch used) were added to subsamples of 100g of grain in flasks. The sorghum was allowed to equilibrate at 4°C for 48h with periodic shaking. Once sealed, the flasks were autoclaved for 20min at 121°C, and then single layers of grain were carefully placed in 9cm sterile plastic Petri dishes on a flow bench. The final aw levels were 0.85, 0.88, 0.91, 0.94, 0.97 and 0.99. All aw values were verified with an AquaLab 3 (Decagon Devices, Inc., WA, USA) with an accuracy of ±0.003 before, during and at the end of the experiment.
Inocula preparation and incubationA. flavus isolates were sub-cultured on potato dextrose agar (PDA) plates and incubated at 25°C for 7d to enable significant sporulation. After incubation, a sterile inoculation loop was used to remove the conidia from the PDA plates. The conidia were suspended in an aqueous solution of 0.05% (w/v) Tween 80. After homogenizing, the spore concentrations were determined using the Thoma cell counting chamber. The suspensions were diluted to adjust the final concentration to vary between 1 and 5×105 spores/ml. Triplicate plates with single layers of sorghum for each water activity and temperature combination were inoculated centrally with a needlepoint load and incubated for 28d. Previous experiments showed that the number of spores inoculated through this technique was 10–100 spores on each inoculation point. The incubation temperatures for all three isolates were 15, 25 and 37°C, and the aw values were 0.85, 0.88, 0.91, 0.94, 0.97 and 0.99. Plates with the same temperature and aw level were stacked in plastic chambers together with 250ml of glycerol/water solution of the same aw to maintain the relative humidity equilibrium in the chambers as constant as possible.
Growth measurement and growth rate calculationThe diameter of each growing colony was determined by measurement of two right-angled rays of the colony. Measurements were recorded daily during the 28d of growth. The growth rate (μ) (mm/d) and growth time (λ) (d) were evaluated for each isolate at each different combination of aw and temperature by plotting the colony diameter extension (mm) against time (d) and fitting the Baranyi2 model using Statgraphics Plus. This model represents the three growth phases (lag, linear, and asymptotic phase). The proposed primary model has the general form of:
Where y is the colony diameter (or radius), y0 is the initial colony diameter (or radius, usually zero), ymax is the maximum colony diameter (or radius) attained (asymptotic value), μmax is the maximum specific growth rate (defined as the slope of the growth curve at the point of inflexion), λ is the lag period (i.e. the intersection of the line defining the maximum specific growth rate with the x-axis), and t is the time.
Aflatoxin B1 production and quantificationAFB1 production was determined after 7 and 14d of incubation under the optimal growth conditions [at 25°C (0.97 and 0.99aw) and at 37°C (0.94, 0.97, and 0.99aw)] and after 21 and 28d of incubation under suitable but not optimal conditions [at 37°C (0.91aw), at 25°C (0.91 and 0.94aw) and at 15°C (0.99aw)] (Table 1). AFB1 was extracted from the sorghum samples (20g) with 100ml of 4% acetonitrile aqueous solution containing potassium chloride (9:1). The mixture was adjusted to a pH of 1.5 with undiluted hydrochloric acid, shaken for 20min and filtered through a Whatman paper. The filtrate was defatted with 100ml of hexane. The solution was shaken for 10min. After separation, the upper phase (hexane) was discarded. This step was repeated with 50ml of hexane. The lower phase was extracted with 50ml of chloroform and 50ml of deionized water, and the solution was shaken for 10min. After separation, the lower phase (chloroform) was collected. The upper phase was re-extracted twice with 25ml of chloroform and 25ml of deionized water. The chloroform extracts were evaporated to near dryness using a rotary evaporator at 40°C. AFB1 was resuspended in 2ml of methanol and evaporated to dryness under nitrogen. Finally, the sample was analyzed with HPLC.
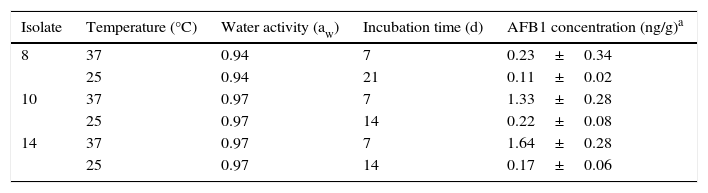
Minimal conditions for aflatoxin B1 (AFB1) production by three Tunisian Aspergillus flavus isolates on sorghum seeds.
| Isolate | Temperature (°C) | Water activity (aw) | Incubation time (d) | AFB1 concentration (ng/g)a |
|---|---|---|---|---|
| 8 | 37 | 0.94 | 7 | 0.23±0.34 |
| 25 | 0.94 | 21 | 0.11±0.02 | |
| 10 | 37 | 0.97 | 7 | 1.33±0.28 |
| 25 | 0.97 | 14 | 0.22±0.08 | |
| 14 | 37 | 0.97 | 7 | 1.64±0.28 |
| 25 | 0.97 | 14 | 0.17±0.06 |
The determination of AFB1 was performed using a Waters (Milford, MA, USA) chromatograph equipped with a reverse phase C18 silica gel column (Water Spherisorb 3μm ODS2 4.6×150mm, Milford, MA, USA), followed by fluorescence detection (λexc 362nm; λem 425nm) (Waters 2475 Florescence Detector, Waters, Milford, MA, USA). The mobile phase consisted of water–acetonitrile–methanol (700:170:170) with a flow rate of 1ml/min. The detection limit (LOD) of the method was 0.075ng/g. The injection volume was 10μl, and the retention time was 8min. Post-column derivatization was achieved using a photochemical reactor for enhanced detection (PHRED) (LCTech UVE, Dorfen, Germany).
Results and discussionPrevious works noted that data obtained regarding mycotoxin production on culture media could not be directly extrapolated to the natural substrates40. Thus, the present study was performed directly on sorghum grains.
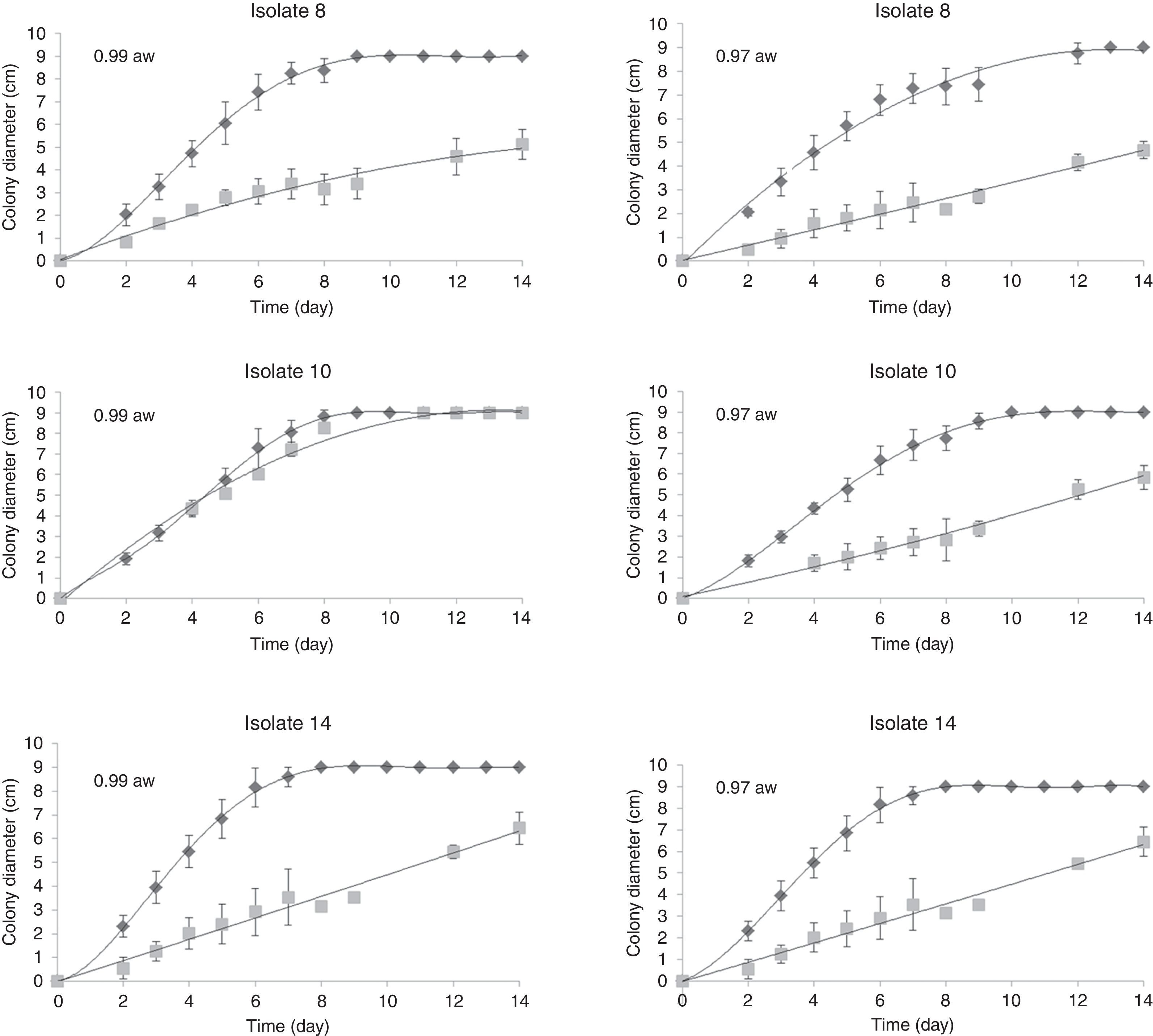
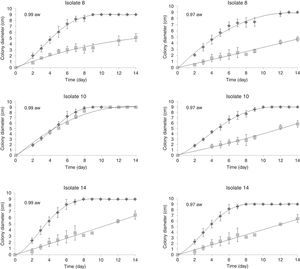
Effects of water activity and temperature on mycelial growthFigure 1 shows an example of the observed colony diameters for A. flavus isolates 8, 10 and 14 at two water activities (0.90 and 0.97) and their respective growth adjusted to the Barany model. Under optimum growth conditions, the colony diameters increased linearly until the colonies reached the edge of the Petri dishes. Under limiting growth conditions, a clear lag time was observed prior to growth (Fig. 2). The Baranyi model was used to determine two growth parameters: the lag phases (d) and the growth rates (mm/d). The percentage of variance explained by the model (R2 coefficient) obtained for the different treatments (combinations) varied between 85.85% and 99.83%, with 31 combinations showing no growth and only two combinations exhibiting 54<90%. The low MSEs (0.017–0.673) and the high R2 values (85.85–99.83%) indicated that the evaluated model could sufficiently determine the growth parameters.
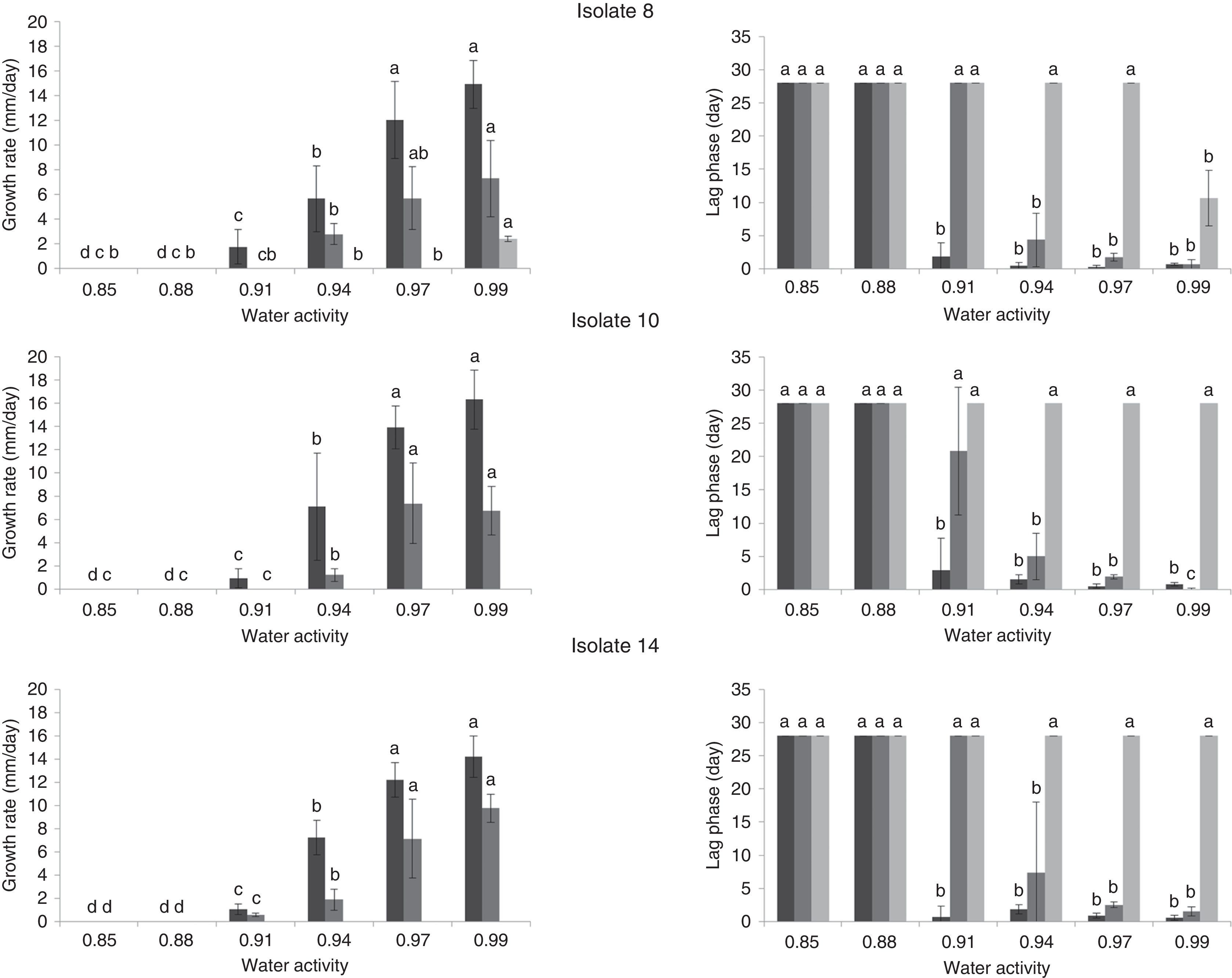
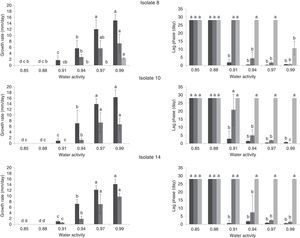
Effect of water activity and temperature on growth rate (mm/day) and lag phases (day) of three A. flavus isolates (8, 10 and 14) on single layers of sterile sorghum grains at different temperatures 37 (
), 25 () and 15°C (). If all letters next to a bar are different from the letters in another bar, there are significant differences among the treatments, if they have a common letter, they are not significantly different (p<0.05).Analysis of variance for the growth rate showed that the single factors aw and temperature and the interactions isolate×temperature, aw×temperature and isolate×aw×temperature had significant influences on the growth rate (p<0.05). On average, the three isolates behaved similarly, as noted by Mohale et al.24 with atoxigenic and toxigenic strains of A. flavus. The combined effects of aw and temperature on the growth rates (mm/d) of the three isolates tested are shown in Fig. 2. The maximum growth rates achieved were 16.29, 14.91 and 14.22mm/d by isolates 14, 8 and 10, respectively, at 37°C and 0.99aw. In this study, the optimum temperature for growth was 37°C for all aw levels tested (Fig. 2). Additionally, 25°C was a very favorable temperature and yielded high growth rates. Similar results for A. flavus isolates were found by Horn23. The extent of seed colonization by Aspergillus section Flavi grew with the increase of water activity and temperature to the optimal conditions of aw=0.96 and 37°C. Samapundo et al.33 showed that A. flavus had optimum growth on corn at 30°C. Schindler et al.28 reported that growth of A. flavus was most favored in wort agar between 29 and 35°C. Temperatures between 25 and 37°C were not tested in our study; however, the mycelial extension of Tunisian A. flavus isolates was significantly higher at 37°C than at 25°C (p<0.05). Figure 2 showed that very short lag phases were encountered at high temperatures (25 and 37°C) and aw (0.97 and 0.99aw). The shorter lag phase period was observed for isolates 10 and 8 at 25°C and for isolate 14 at 37°C. Samapundo et al.33 showed that an increase in temperature from 30 to 37°C resulted in a decrease in the colony growth rate and an increase in the lag phase.
No growth was detected at 15°C at any of the aw conditions tested for isolates 10 and 14; however, isolate 8 grew at 0.99aw. The minimum growth rate observed was 0.9mm/d, which was calculated for isolate 14 at 37°C and 0.91aw. No growth was detected for isolates 10 and 14 at 25°C and 0.91aw. However, at 37°C all isolates grew at 0.91aw. No growth at 0.85 and 0.88aw was detected after 28d at all tested temperatures. However, Pitt and Miscamble11 reported that the minimum water activity for growth of A. flavus was between 0.80 and 0.83. The discrepancy in these results may be due to differences in the media used and the availability of nutrients. Some components of the pericarp layer of the grain, such as tannins and high levels of phenol-based pigments, and some antifungal proteins can affect fungal growth11. Similar studies have also indicated that the availability of nutrients may affect the chances of growth at marginal aw values13.
Maximum growth rates were observed for the three isolates at 25 and 37°C at 0.97 and 0.99aw. Additionally, the A. flavus isolates assayed grew faster at 0.99aw than at 0.97aw at 25 and 37°C, with the exception of isolate 14, where the optimum water activity for growth was 0.97aw at 25°C. Similar results were reported by Marin et al.18 for A. flavus grown on maize extract agar, where the optimal aw for growth was 0.994. However, the optimum water activity for growth in some studies was somewhat different from the conditions demonstrated by our data. For instance, Horn23 found that the optimum water activity for growth was 0.96aw. These differences can be attributed to intraspecific and regional variability among isolates.
Effects of water activity and temperature on aflatoxin B1 productionAFB1 was the only metabolite considered in the data analysis because AFB2 was rarely detected and only in trace amounts whereas aflatoxins G1 and G2 were never detected. AFB1 has been reported to be the major AFB in natural substrates such as sorghum1,9,10. A similar situation was reported by Giorni et al41.
Analysis of variance of the effects of aw and temperature on AFB1 production by the three A. flavus isolates revealed that the single factors aw and temperature and the interactions temperature×isolates, aw×incubation time and temperature×incubation time had a significant effect on AFB1 production after 7 and 14d of growth on sorghum seeds (p<0.05). After 21 and 28d, only the single factors aw and temperature and the interaction between water activity and isolates significantly affected AFB1 accumulation (p<0.05).
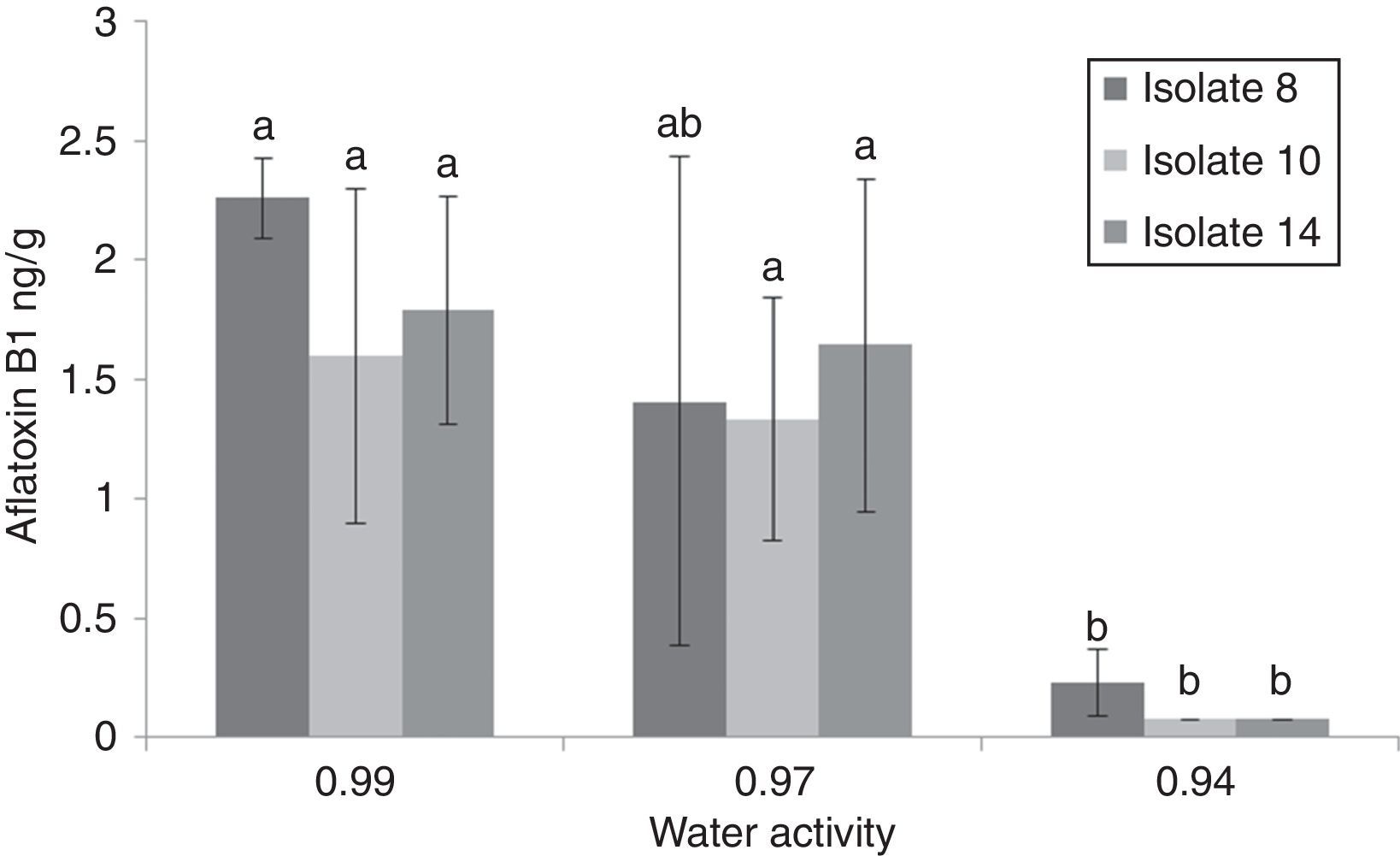
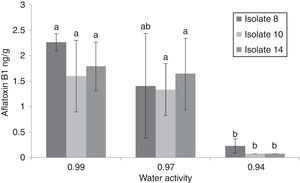
The maximum accumulation of AFB1 (2.26ppb) was observed for isolate 8at 37°C and 0.99aw after 7d of incubation (Fig. 3). Generally, at 37°C and 25°C AFB1 accumulation grew with increasing water activities (0.97 and 0.99aw). No significant differences were found between 0.99 and 0.97aw (p<0.05) at 37°C after 7d of incubation. At 37°C, significantly higher amounts of AFB1 (p<0.05) were produced by the three A. flavus isolates at 0.97 and 0.99aw. However, Montani et al.32 found that the most favorable aw for aflatoxin production was 0.90aw at all incubation times studied. In this study, the only isolate that produced AFB1 at 0.94 was isolate 8, which produced 0.23ng/g at 37°C after 7d of incubation and 0.105ng/g at 25°C after 21d. To determine the minimum aw that allowed aflatoxin production, we tested aw values of 0.85, 0.88 and 0.91aw. AFB1 was not detected at 25 and 37°C, whereas growth occurred at 0.91aw and 37°C. AFB1 was detected under a narrower range of water activity than the range that allowed growth (0.94 and 0.99aw). Consequently, it may be possible to avoid aflatoxin accumulation from these strains by storing the commodities at “safe” water activity levels (≤0.91aw). At 15°C, AFB1 was not detected (Table 1). Hunter31 proposed 0.84aw as the minimal value for aflatoxin production in corn, and Astoreca et al.,26 proposed 0.83 as the minimum value in corn extract medium.
Comparison aflatoxin B1 (AFB1) production by 3 Aspergillus flavus isolates after 7 days of incubation on sorghum seeds at different water activities (0.94, 0.97 and 0.99) (A, B, C, D). If all letters next to a bar are different from the letters in another bar, there are significant differences among the treatments, if they have a common letter, they are not significantly different (p<0.05).
Several researchers reported a different behavior of the A. flavus isolates regarding temperature, with optimum values varying from 25 to 30°C regardless of the media5,12,25. For instance, Schindler et al.28, reported that maximal production of aflatoxins on wort media occurred at 24°C, whereas the optimal temperature for AFB1 production was 35°C.
The incubation period that allowed higher AFB1 accumulation depended on the temperature, water activity and isolate tested. At the optimal temperature (37°C) and 0.99aw, all isolates required 7d to reach maximum AFB1 production; no AFB1 production was detected after 14d under the same conditions. At water activities above 0.94aw, AFB1 accumulated at 37°C was lower after 14d of incubation on sorghum grains than after 7d (p<0.0001). The results showed that the optimal aw for AFB1 accumulation at 25°C was 0.99aw. At 25°C, the amounts of AFB1 detected at 0.97 and 0.99aw after 14d of incubation increased compared to the amounts produced after 7d of incubation, with the exception of isolate 8 at 0.99aw, whose AFB1 accumulation was higher after 7d of incubation (p<0.001). AFB1 decrease following the peak of maximum production was observed under the optimal conditions (37°C and 0.97–0.99aw). The decrease in AFB1 concentration may be explained by its degradation caused by the mold itself. The detection of AFB1 after 21d and 28d of incubation showed that only isolate 8 produced AFB1 at 0.94aw and 37°C and 25°C after 7 and 21d, respectively. A. flavus species comprise a high number of isolates with certain genetic variability, which explains why the expression of genes involved in the aflatoxin metabolic pathway is dependent on the environmental conditions.
In our study, maximum AFB1 production could not be linked to a specific time point after inoculation, but overall 7d of incubation was generally sufficient for A. flavus to achieve important AFB1 accumulation under the optimal conditions for AFB1 production (37°C and 0.97–0.99aw). Nevertheless, the optimum incubation period and temperature required for maximum AFB1 production varied, probably due to the set of conditions tested, the nature and composition of the substrate and intraspecific and regional variability among isolates.
In this study, maximal growth was correlated with maximal aflatoxin production (0.99aw and 37°C). However, other studies have shown that good growth may occur without any production of aflatoxins. For example, Rabie and Smalley15 demonstrated that the maximal production of aflatoxins occurred at 24°C, but the maximal growth of A. flavus isolates occurred at 29 and 35°C.
This is the first study concerning the effect of water activity and temperature on A. flavus growth and AFB1 production on sorghum grains.
The optimal conditions for mycelial growth and AFB1 production were 0.99aw and 37°C. There was neither A. flavus growth nor AFB1 production at 0.85 and 0.88aw at all temperatures investigated. Consequently, aflatoxin accumulation could be avoided by storing sorghum at low water activity levels (≤0.91aw). Our results showed that A. flavus isolated from Tunisian sorghum was able to grow in a wide range of water activities (0.91–0.99aw) and temperatures (15–37°C), however, the production of AFB1 occurred at a narrower range of water activities (0.94–0.99aw) and temperatures (25–37°C). Within the range of aw evaluated in this study, 0.94 was the limiting level for AFB1 production. The investigation of trends for growth and AFB1 production at different temperatures and water activities in sorghum seeds can provide important information concerning the contamination of sorghum by AFB1. Our study contributes to the understanding of the ecology and physiology of Tunisian A. flavus isolates.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank M. Prim for her encouragement and technical assistance. The authors are grateful to the European Union (MYCORED KBBE-2007-2-5-05 project) and Tunisian Government for financial support.