The aim of this study was to evaluate the antifungal activity (in vitro) of thymol and carvacrol alone or in mixtures against Fusarium verticillioides and Rhizopus stolonifer, and to obtain primary growth models. Minimal inhibitory concentration (MIC) was evaluated with fungal radial growth with thymol or carvacrol concentrations (0–1600mg/l). Mixtures were evaluated using concentrations below MIC values. Radial growth curves were described by the modified Gompertz equation. MIC values of carvacrol were 200mg/l for both fungi. Meanwhile, MIC values of thymol were between 500 and 400mg/l for F. verticillioides and R. stolonifer, respectively. A synergistic effect below MIC concentrations for carvacrol (100mg/l) and thymol (100–375mg/l) was observed. Significant differences (p<0.05) between the Gompertz parameters for the antimicrobial concentrations and their tested mixtures established an inverse relationship between antimicrobial concentration and mycelial development of both fungi. Modified Gompertz parameters can be useful to determine fungistatic concentrations.
El objetivo de este trabajo fue evaluar la actividad antifúngica in vitro del timol y del carvacrol, solos o en mezclas, contra Fusarium verticillioides y Rhizopus stolonifer, y obtener modelos primarios de crecimiento. Se evaluó la concentración inhibitoria mínima (CIM) con el crecimiento radial, se ensayaron concentraciones de timol y carvacrol de 0 a 1.600mg/l. Las mezclas se evaluaron utilizando concentraciones por debajo de los valores de CIM. Las curvas de crecimiento radial fueron descritas por la ecuación de Gompertz modificada. Se obtuvieron los siguientes valores de CIM: carvacrol, 200mg/l para las 2 especies; timol, 500mg/l y 400mg/l para F. verticillioides y R. stolonifer, respectivamente. Se observó un efecto sinérgico a concentraciones inferiores a las CIM para el carvacrol (100mg/l) y el timol (100-375mg/l). Hubo diferencias significativas (p<0,05) entre los parámetros de crecimiento de Gompertz; se estableció que existe una relación inversa entre la concentración de los antimicrobianos y el desarrollo del micelio de ambos hongos.
Fusarium spp. and Rhizopus spp. are widespread and ubiquitous in the environment. They can contaminate food before harvest or under post-harvest conditions and are considered food spoilers. Some Fusarium species can produce mycotoxins, decreasing the commercial value of the affected products1.
Fungal contamination, colonization and infection of plants are initiated by contact of the host with conidia (spores) and subsequent conidial germination. Germination and initiation of infections involve biochemical activities with an increase in metabolism and induction of morphological changes. Fungal survival and growth in food may lead to spoilage and toxin formation. These metabolites are structurally diverse compounds and represent an important category of natural toxins that can affect humans and result in economic losses worldwide12.
For years, synthetic chemical additives were efficient to control food fungal growth; however, these products represent a potential hazard to human health. Currently, a lot of studies on food preservation by natural compounds are being carried out8. Many antimicrobial compounds from plants have been identified; several publications reported the antifungal activity of some phenolic components of essential oils such as thymol and carvacrol. Natural isopropyl cresols, carvacrol (5-isopropyl-2-methylphenol), and thymol (2-isopropyl-5-methylphenol) are the major components of oregano (Origanum spp.) and thyme (Thymus spp.) essential oils. Some researchers have pointed out the antimicrobial activity against bacteria, molds, and yeast11 of these natural extracts. Carvacrol and thymol are Generally Recognized as Safe (GRAS) food additives, and are used as flavoring agents in baked goods, sweets, beverages, and chewing gum3.
Food preservation trends indicate the use of new chemical preservatives simultaneously in mixtures to keep food safety and quality at lower antimicrobial doses1. These mixtures provide a wider range of increasing activity against different pathogenic microorganisms, or act on several points inside cells, which can enable a better control compared to individual agents13. Moreover, in order to evaluate antimicrobial action, predictive microbiology models are valuable. Different primary models describe either germination or mycelium growth kinetics of various fungal species on food products15. The modified Gompertz equation is a suitable predictive tool applied in nonlinear growth curves that describe quantitative parameters such as growth, lag phase and fungal growth rate12.
Therefore, the aim of this study was to evaluate the antifungal activity (in vitro) of thymol and carvacrol alone or in mixtures against Fusarium verticillioides and Rhizopus stolonifer species, and to obtain predictive models of growth.
Microorganisms and preparation of cultures: F. verticillioides and R. stolonifer were obtained from the Facultad de Ciencias Químicas (Benemérita Universidad Autónoma de Puebla) collection. The microorganisms were maintained on Petri dishes containing sterilized potato-dextrose agar (PDA Merk, Mexico City, Mexico) and incubated in a dark environment at 28°C for 5–6 days until fungal growth was observed. Fungal structures (conidia and mycelia) were observed using a Zeiss Primo Star microscope (Carl Zeiss AG, Göttingen, Germany) and identified according to taxonomic keys4.
Minimum Inhibitory Concentration (MIC): thymol or carvacrol (Sigma–Adrich, Milwaukee, WI, USA) were mixed (using a vortex shaker) with sterilized PDA medium to achieve final concentrations of 100, 150, 200, 400, 800 and 1600mg/l (concentrations were selected according to reference1). Agar solutions were poured into sterile petri dishes. Fungal spores were obtained by pouring 9ml of sterile physiological water (0.90% w/v of NaCl) on the agar plate surface previously inoculated with each mold, followed by gentle scraping using a sterile rake to remove the maximum quantity of spores. Spore suspensions were transferred into sterile tubes. The number of spores present in the suspension was determined using a hemocytometer and an optical microscope (Zeiss Primo Star, Göttingen, Germany), and expressed as number of spores per milliliter (spores/ml). Suspensions were serially diluted to approximately 1000spores/ml. Finally, plates were inoculated with 10μl of spore suspension in the center of the plate and were incubated at 28°C; radial growth was measured every 12h during 84–96h. A growth control was prepared in parallel to ensure that viable organisms were present. Every test was performed in triplicate. MIC values were determined as the lowest concentration at which no growth occurred3.
Antimicrobial mixtures: Mixtures were prepared using concentrations below the MIC value; the evaluation was conducted in accordance with the procedure for the individual antimicrobial assay. In order to evaluate the effects of antimicrobial mixtures a checkerboard array was used; the MIC values for individual antimicrobial or their mixtures were defined as the minimal concentration required to inhibits fungal growth. The MIC data were transformed to fractional inhibitory concentration (FIC) (Eqs. (1) and (2)):
The FICIndex (Eq. (3)) for mixtures was calculated with the FIC for individual antimicrobials7, a FICIndex near 1 indicates additive effect, whereas <1 indicates synergism and >1 indicates antagonism:
Fungal growth modeling: All growth curves were fitted using the modified Gompertz model (Eq. (4))5
where Dt is the average diameter at time t (h), Do is the average diameter at initial time (0.02cm), A is the maximum fungal growth achieved during the stationary phase, υmax is the maximum specific growth rate (1/h), λ is the lag time (h), and e=exp(1); nonlinear regression was performed using the KaleidaGraph program (3.21 Synergy Software, Reading, PA., U.S.A.).Statistical analysis: Growth parameters were analyzed by ANOVA. Significant values were subjected to mean analysis by the Tukey's test, using a confidence level of 95%. Statistical analyses were performed using Minitab15 (LEAD Technologies Inc., NJ, U.S.A.).
MIC values of thymol and carvacrol and mixtures of these compounds: thymol and carvacrol, which are isomer molecules, showed antimicrobial activity against both fungi. The MIC values for F. verticillioides for carvacrol was 150mg/l and for thymol 400mg/l. The MIC values for R. stolonifer for carvacrol was 200mg/l and for thymol 800mg/l. Mixtures were effective in 7 of 9 combinations for F. verticillioides and in 6 of 9 combinations for R. stolonifer (Tables 1 and 2). Only one of the seven inhibitory mixtures showed synergistic action (100mg/l of thymol and 100mg/l of carvacrol) against F. verticillioides (FICIndex 0.92) while four of the six inhibitory mixtures showed synergistic action against R. stolonifer (FICIndex average 0.85±0.11). Moreover, the mixture containing 375mg/l of thymol and 50mg/l of carvacrol showed the lowest FICIndex value (0.72).
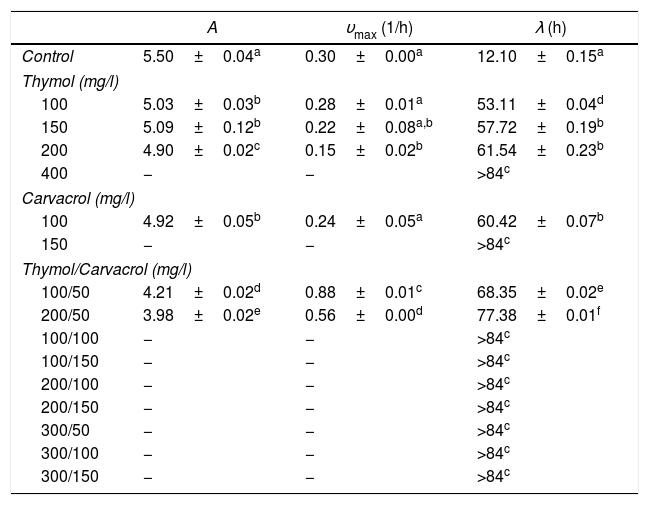
Modified Gompertz model parameters+ (mean±standard deviation) for F. verticillioides growth curves subjected to selected concentrations of thymol and carvacrol
| A | υmax (1/h) | λ (h) | |
|---|---|---|---|
| Control | 5.50±0.04a | 0.30±0.00a | 12.10±0.15a |
| Thymol (mg/l) | |||
| 100 | 5.03±0.03b | 0.28±0.01a | 53.11±0.04d |
| 150 | 5.09±0.12b | 0.22±0.08a,b | 57.72±0.19b |
| 200 | 4.90±0.02c | 0.15±0.02b | 61.54±0.23b |
| 400 | − | − | >84c |
| Carvacrol (mg/l) | |||
| 100 | 4.92±0.05b | 0.24±0.05a | 60.42±0.07b |
| 150 | − | − | >84c |
| Thymol/Carvacrol (mg/l) | |||
| 100/50 | 4.21±0.02d | 0.88±0.01c | 68.35±0.02e |
| 200/50 | 3.98±0.02e | 0.56±0.00d | 77.38±0.01f |
| 100/100 | − | − | >84c |
| 100/150 | − | − | >84c |
| 200/100 | − | − | >84c |
| 200/150 | − | − | >84c |
| 300/50 | − | − | >84c |
| 300/100 | − | − | >84c |
| 300/150 | − | − | >84c |
+ A: maximum fungal growth in the stationary phase; υmax: maximum specific growth rate; λ: lag phase.
− No growth. Means followed by a different superscript letter within a column for each are significantly different (p<0.05).
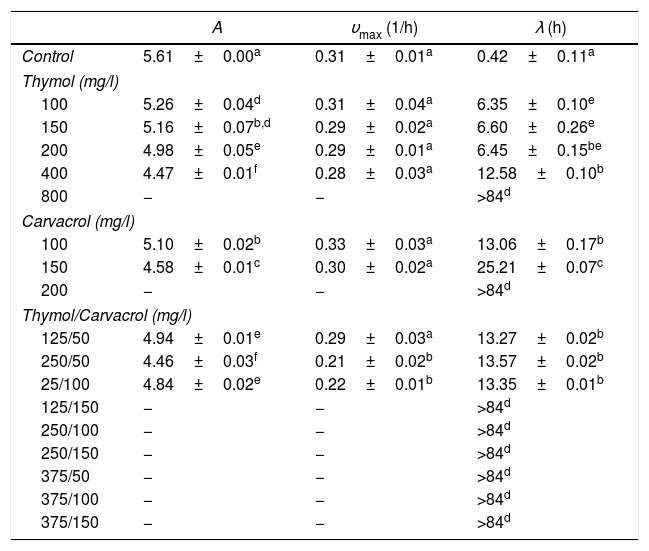
Modified Gompertz model parameters+ (mean±standard deviation) for R. stolonifer growth curves subjected to selected concentrations of thymol and carvacrol
| A | υmax (1/h) | λ (h) | |
|---|---|---|---|
| Control | 5.61±0.00a | 0.31±0.01a | 0.42±0.11a |
| Thymol (mg/l) | |||
| 100 | 5.26±0.04d | 0.31±0.04a | 6.35±0.10e |
| 150 | 5.16±0.07b,d | 0.29±0.02a | 6.60±0.26e |
| 200 | 4.98±0.05e | 0.29±0.01a | 6.45±0.15be |
| 400 | 4.47±0.01f | 0.28±0.03a | 12.58±0.10b |
| 800 | − | − | >84d |
| Carvacrol (mg/l) | |||
| 100 | 5.10±0.02b | 0.33±0.03a | 13.06±0.17b |
| 150 | 4.58±0.01c | 0.30±0.02a | 25.21±0.07c |
| 200 | − | − | >84d |
| Thymol/Carvacrol (mg/l) | |||
| 125/50 | 4.94±0.01e | 0.29±0.03a | 13.27±0.02b |
| 250/50 | 4.46±0.03f | 0.21±0.02b | 13.57±0.02b |
| 25/100 | 4.84±0.02e | 0.22±0.01b | 13.35±0.01b |
| 125/150 | − | − | >84d |
| 250/100 | − | − | >84d |
| 250/150 | − | − | >84d |
| 375/50 | − | − | >84d |
| 375/100 | − | − | >84d |
| 375/150 | − | − | >84d |
+ A: maximum fungal growth in the stationary phase; υmax: maximum specific growth rate; λ: lag phase.
− No growth. Means followed by a different superscript letter within a column for each are significantly different (p<0.05).
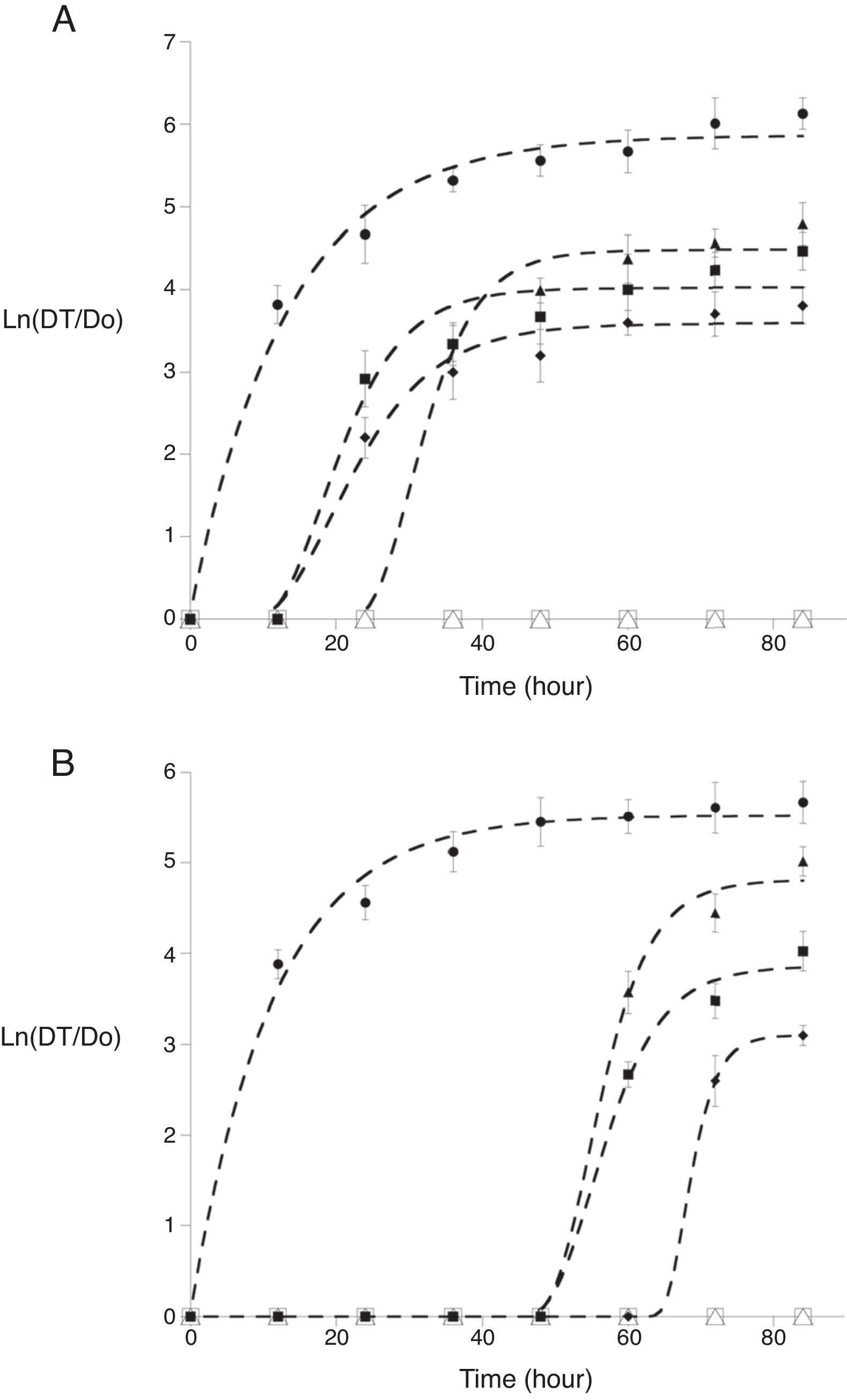
Fungal growth modeling: Although the modified Gompertz equation is a primary predictive model whose parameters adequately described fungal growth (mean coefficient of determination 0.991±0.04), the statistical analysis of modified Gompertz parameters showed differences (p<0.05) in maximum fungal growth (A) and lag phase (λ). Moreover, the increase of both antimicrobial concentrations increases λ values. The modified Gompertz parameters (A and λ) for F. verticillioides showed significant differences (p<0.05) with thymol and carvacrol individually and in mixtures (100/50 and 200/50thymol/carvacrol mg/l). Some combinations under MIC values affect radial growth and increase the lag phase (Fig. 1A). For R. stolonifer three thymol/carvacrol combinations (125/50, 250/50 and 125/100mg/l) showed the same effect in growth parameters (Table 2), especially in the lag phase (p>0.05) with a fungistatic effect, as single compound applications of 200mg/l of thymol and 100mg/l of carvacrol (Fig. 1B). For both fungi an inverse relationship between antimicrobial concentration and mycelial development is established; carvacrol can extend the lag phase up to 5 and 60h (for F. verticillioides and R. stolonifer respectively) compared to control.
Effect of carvacrol, thymol, and his mixtures at selected concentrations [0 (●), 150 carvacrol (▴), 200 carvacrol (Δ), 400 thymol (■), 800 thymol (□), and 250/50 thymol/carvacrol (♦) mg/l] on R. stolonifer growth (A) and on F. verticillioides (B) [0 (●), 100 carvacrol (▴), 150 carvacrol (Δ), 200 thymol (■), 400 thymol (□), and 200/50 thymol/carvacrol (♦) mg/l] Dt is the average colony diameter at time t and Do is the average colony diameter at initial time, fitted (---) with the modified Gompertz model.
Our results demonstrate that mixtures of thymol and carvacrol inhibit the studied mold strains, with lower concentrations than those needed when the antimicrobial is utilized individually. The use of antimicrobial mixtures provides a wider range of activity, which can enable a better control of the evaluated molds under in vitro conditions, as it has been reported when results were compared with the use of an individual antimicrobial agent8. Similar results of MIC values for F. verticilliodes were obtained6, which showed that thymol and carvacrol affect radial growth and conidial production and germination. Moreover, similar MIC values were obtained when thymol and carvacrol were used against F. oxysporum (350mg/ml and 150mg/ml) and R. oryzae (500mg/ml and 250mg/ml)1. There are no studies on R. stolonifer inhibition with natural antimicrobial mixtures. Thymol and carvacrol have a hydroxyl group at different locations on the phenolic ring that increases the ability to dissolve the microbial membrane causing the loss of macromolecules of the cell inhibiting fungal conidial germination10. Moreover, they can change pH homeostasis, K+ gradient, resulting water imbalance, intracellular ATP depletion and cell death2. These isomers affect radial growth, production, and conidial germination and cause morphological changes9. Thymol and carvacrol inhibit ergosterol biosynthesis which consequently affects cell membrane integrity1. These cell modifications affect morphology, such as lack of sporulation, loss of pigmentation, irregular development of conidiophores and distortional hyphae3,5.
Antimicrobial interaction mechanisms are less known. There are some hypotheses that mention that phenolic mixtures could increase the number, size or duration of membrane pores by binding phenolic compounds with different proteins or enzymes embedded in the cell membrane14. A synergistic effect would be achieved when two components probably disintegrate the lipid membrane and make it easier for the other component to enter the cytoplasm13.
Primary models are suitable to predict fungal growth and may be useful for research purposes, although it is possible to be a routine measurable parameter on food analysis. Modified Gompertz parameters can be useful to determine fungistatic concentrations (2 of 9 mixtures for F. verticillioides and 3 of 9 for R. stolonifer). Fungal growth rate and lag time are parameters, which subsequently may be used for a secondary modeling if growth curves for different constant conditions are available15. There are few reports that describe the effect of natural antimicrobials using mathematical models; fungal growth parameters provide information that can be used to assess the response of other fungal strains as well as to evaluate antifungal agents in additional systems or foods.
Ethical disclosuresProtection of human and animal subjectsThe authors state that no human or animal experiments have been performed for this research.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
This study was partly funded by the Facultad de Ciencias Químicas of the Benemérita Universidad Autónoma de Puebla and the National Council for Science and Technology of México (CONACyT).







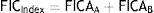
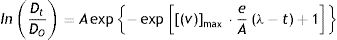
![Effect of carvacrol, thymol, and his mixtures at selected concentrations [0 (●), 150 carvacrol (▴), 200 carvacrol (Δ), 400 thymol (■), 800 thymol (□), and 250/50 thymol/carvacrol (♦) mg/l] on R. stolonifer growth (A) and on F. verticillioides (B) [0 (●), 100 carvacrol (▴), 150 carvacrol (Δ), 200 thymol (■), 400 thymol (□), and 200/50 thymol/carvacrol (♦) mg/l] Dt is the average colony diameter at time t and Do is the average colony diameter at initial time, fitted (---) with the modified Gompertz model. Effect of carvacrol, thymol, and his mixtures at selected concentrations [0 (●), 150 carvacrol (▴), 200 carvacrol (Δ), 400 thymol (■), 800 thymol (□), and 250/50 thymol/carvacrol (♦) mg/l] on R. stolonifer growth (A) and on F. verticillioides (B) [0 (●), 100 carvacrol (▴), 150 carvacrol (Δ), 200 thymol (■), 400 thymol (□), and 200/50 thymol/carvacrol (♦) mg/l] Dt is the average colony diameter at time t and Do is the average colony diameter at initial time, fitted (---) with the modified Gompertz model.](https://static.elsevier.es/multimedia/03257541/0000005000000001/v1_201803060440/S0325754117301001/v1_201803060440/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
