Honey is a product used as a natural sweetener and in several regions of Mexico and other countries it is also used as a therapeutic agent. Microbiological contamination of honey can occur during its extraction and handling. Due to the use and consumption of honey we highlighted here the importance of the assessment of its microbiological quality. One thousand nine hundred twenty samples obtained from 8 honey-producing states from Mexico were analyzed. From these samples, 40.5% (777/1920) did not comply with the NMX-036-NORMEX-2006 specification. Forty five percent (777/1920) of the samples did not comply with the mesophilic aerobic microorganism specification, neither did 17% (327/1920) of the samples with the specification for molds and 18.1% (348/1920) with the specification for yeasts. With regard to coliform bacteria, the samples contained less than 3 NMP/g. Two percent of the samples contained lactic acid bacteria (LAB), Clostridium perfringens was observed in amounts of more than 100CFU/g. None of the samples from the different states contained more than 100CFU/g of Staphylococcus aureus; Salmonella spp. was absent in all samples. It is important to avoid contamination sources and implement good hygienic practices in order to maintain and improve the quality of Mexican honeys since a large percentage of them are intended for export. If these honeys are intended for therapeutic use, it is necessary to ensure that they comply with all quality parameters and to apply specific treatments that guarantee the removal of any pathogen that may represent a risk to the patients's health.
La miel es un producto que se usa como edulcorante natural y en algunas regiones de México y otros países, como agente terapéutico. La contaminación de la miel con microorganismos se puede presentar durante la extracción y el manejo. Dado el uso y consumo de la miel, se consideró importante evaluar su calidad microbiológica. Se trabajó con 1.920 muestras de miel obtenidas de 8 estados productores en México. De las muestras de miel analizadas, el 40,5% (777/1.920) estuvo fuera de las especificaciones de la NMX-036-NORMEX-2006. Todas esas muestras no cumplían con las especificaciones referidas a mesofílicos aerobios, en tanto que el 17% (327/1920) no cumplían las relativas a mohos y el 18,1% (348/1920), las fijadas para levaduras. En lo que se refiere a los organismos coliformes, las muestras contenían < 3 NMP/g. Se encontró que el 2% contenía BAL; Clostridium perfringens se puso en evidencia en el 12%, con más de 100 UFC/g. En el caso de Staphylococcus aureus, ninguna muestra estuvo por arriba de 100 UFC/g. Salmonella spp. no se detectó en ninguna de las muestras analizadas. Es importante evitar las fuentes de contaminación y observar buenas prácticas de higiene para mantener y mejorar la calidad de las mieles mexicanas, ya que un buen porcentaje de ellas están destinadas a la exportación. Si estas van a ser destinadas a un uso terapéutico es necesario asegurarse de cumplir con los parámetros de calidad y dar tratamientos específicos que aseguren la eliminación de algún patógeno que pueda poner en riesgo la salud del paciente que utilice la miel.
Honey is a product used as sweetener and in some regions of Mexico and other countries it is also used as a therapeutic agent. It has a high sugar content, mainly fructose and glucose, low water activity (aw=0.50–0.60), and a high osmotic potential with a humidity lower than 18%, a high acidity and low pH (3.4-6-1)17. In our country, Apiculture has a high social and economic value. Forty five thousand producers depend on it, which accounts for 19 million of beehives and allow Mexico to be placed as the fifth producing country and as the third exporting country in the world27. Several studies have shown the presence of substances with antimicrobial activity such as hydrogen peroxide and several phytochemical compounds such as flavonoids, phenols, organic acids like cinnamic acid, methyl syringate, and methylglyoxal, which limit the amount of microorganisms present in honey and allow only those that manage to survive to remain viable4,11,36.
The microbial characteristics of honey are a consequence of the intrinsic biota of bees, since honey is the product of the nectar of different flowers of distinct species that is kept during a different amount of time in their honey stomach without getting contaminated since it never gets in touch with the gut of the bee3. Contamination of honey with other microorganisms can occur during extraction and handling15. The following are some of the primary sources of microbial contamination in honey: the digestive tracts of honey bees, dust, air, dirt and flowers. Secondary sources of microbes in honey are likely to be the same as for other foods30.
Different types of microorganisms such as Actinetobacter spp., Bacillus spp., Clostridium spp., Corynebacterium spp., Pseudomonas spp. are bacteria commonly found in soil. Saccharomyces and Torula yeasts can be found in high-moisture sugars, and Leuconostoc mesenteroides has been found in sugar refineries. Brochothrix spp., Citrobacter spp., Enterobacter spp., Erwinia spp., Flavobacterium spp., Lactobacillus spp., Lactococcus spp., Leuconostoc spp., Listeria spp. and Pediococcus spp. are found in plants and plant products30.
Among the sporulating bacteria, different species of Bacillus have been reported as well as Clostridium botulinum spores and in a lesser proportion those of Clostridium perfringens. C. botulinum can occasionally cause infant botulism in children less than one year of age; C. perfringens can cause gastrointestinal alterations or gas gangrene in deep wounds, a possibility that is considered because of the current use of honey as a healing agent in wounds23. There is no Official Mexican Standard, however, in 2006 a non-mandatory standard was elaborated (NMX-036-NORMEX-2006)19 establishing that the presence of no more than 1000CFU/g of nonpathogenic bacteria and up to 100CFU/g of yeast and molds should be accepted.
Mexico is one of the main honey producers worldwide; in 2010 it was the third exporter with 56 thousand tons and in 2012 it raised to 86 thousand tons, being the state of Yucatán, the main producer, followed by Campeche and Jalisco with a total aggregate of almost 40% of the national production among these three states25–27.
As a result and due to the use and consumption of honey, we considered it important to assess the microbiological quality of honey by studying the presence of aerobic mesophilic bacteria, coliforms, molds, yeasts, lactic acid bacteria, Staphylococcus aureus, Salmonella spp. and C. perfringens in samples from several producing regions in Mexico.
Materials and methodsSampling sitesA total of 1920 samples of honey obtained from 8 producing states in Mexico (Mexico City, the States of: México, Yucatán, Jalisco, Morelos, Chihuahua, Oaxaca and Michoacán) were analyzed during two years (2010–2011). Two hundred forty samples from each state were assessed, handling 80 samples a month (10 of each state).
Sample handlingOne thousand grams of honey were collected from the sites of process in clean containers, which were shipped in ice boxes at approximately 10°C by air or ground transportation to the laboratory for their analysis.
Microbiological analysisTwenty five (25) g of each sample were added to 225ml of 0.1% peptone water homogenized on a STOMACHER 400 Circulator (Seward®, London), and decimal dilutions were made up to 10−6.
Each dilution was plated on potato dextrose agar acidified with tartaric acid using the pour plate technique and incubated at 25°C during 5 days for molds and at 35°C during 48h for yeasts21. Standard method agar was used for the quantification of aerobic mesophilic (MA) bacteria with an incubation period of 48h at 35°C20. Coliform organisms (OC) were assessed by the most probable number (MPN) using 101 to 103 dilution by the 3,3,3 series on lactose broth; 1.0ml of each dilution was transferred to the broth and incubated at 35°C. Gas formation was observed after 24 and 48h. A sample from the positive tubes was inoculated in 2% brilliant green bile lactose, incubated at 35°C for 24–48h and afterwards, tables were consulted in order to establish the MPN/g on each sample of honey22. For the determination of lactic acid bacteria (LAB), samples were inoculated onto de Man, Rogosa, and Sharpe agar (MRS)5 containing 1% of glucose and peptonized milk agar by spreading the plate and incubated at 37°C with low CO2 tension (Labcon Co®), for 48h. For species identification several tests were performed, such as Gram staining, determination of catalase and oxidase activities, carbohydrate fermentation patterns (glucose, lactose,sucrose to detect gas production), motility and ability of strains to grow at different temperatures, pH and salt concentrations5,28,32.
S. aureus samples were inoculated onto Baird-Parker media by the spreading technique and incubated for 48h at 35°C. Plates containing between 15 and 150 typical colonies of S. aureus were selected for coagulase and thermonuclease tests to confirm this microorganism22. For the determination of Salmonella spp., 25g of each honey sample were added to 225ml of lactose broth, incubated for 24h at 35°C, and 0.1ml was transferred to 10ml of Rappaport-Vassiliadis media and 1ml to tretrathionate broth, incubated at 37 and 42°C respectively for 24h; the obtained cultures were streaked onto MacConkey, xylose lysine deoxycholate and brilliant green agar plates, incubated at 35°C for 24h and the biochemical and serological identification was performed22.
For the isolation of C. perfringens we used the methodology outlined in the BAM-FDA24. C. perfringens ATCC 10239 was used as a positive control. Using an aseptic technique, 25g of honey sample were placed into a sterile blender jar, 225ml peptone dilution fluid (1:10 dilution) were added, and the mixture was homogenized for 1–2min at low speed, avoiding aeration to obtain a uniform homogenate. Using the 1:10 dilution described above, serial dilutions were made from 101 to 106 by spreading 0.1ml of each dilution using a sterile glass rod spreader over previously poured plates of TSC agar containing egg yolk emulsion. Plates were overlaid with 10ml of TSC agar without egg yolk emulsion. Anaerobic conditions were established, and the jars were placed in a 35°C incubator for 20–24h. Plates containing 20–200 black colonies were selected for counting (C. perfringens colonies in egg yolk medium are black with a 2–4mm opaque white zone surrounding the colony, as a result of lecithinase activity). Black colonies were counted and the number of Clostridium cells per gram of food was calculated. Plates were saved for identification tests. All was done in triplicate. Ten typical C. perfringens colonies were selected and each of them was inoculated into a tube of freshly deaerated and cooled thioglycollate broth. Tubes were incubated in a standard incubator for 18–24h at 35° C. Each culture was examined by Gram staining and checked for purity. A 1-ml sample of each actively growing fluid thioglycollate culture was inoculated into modified iron-milk medium, and then incubated at 46°C in a water bath. After 2h, the cultures were checked every 60min for stormy fermentation24. When this test was positive, a complete test was carried out. Afterwards, biochemical identification was performed24.
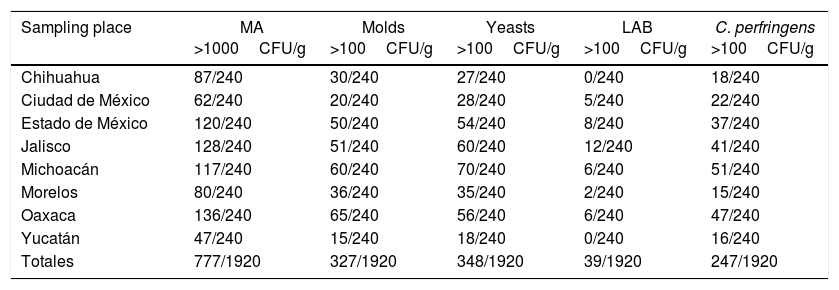
ResultsFrom the 1920 honey samples analyzed, 40.5% (777/1920) did not meet the specification for NMX-036-NORMEX-2006. With regard to the indicator groups for mesophilic aerobic bacteria, 40.5% (777/1920) of the samples did not comply with meet the normative standards. Table 1 shows the number of analyzed samples that did not meet the specification established by the standard, which corresponds to 56.7% of the honey samples from Oaxaca, 53.3% from Jalisco, 50% from the State of Mexico, 48.8% from Michoacán, 36.3% from Chihuahua, 33.3% from Morelos, 25.8% from Mexico city and 19.6% from Yucatán. The bacterial content ranged from 1200 to 9400CFU/g.
With regard to molds, 17% (327/1920) of the analyzed samples were outside the limits established by the standard while in yeasts, 18.1% (348/1920) of the samples showed the same behavior. CFU for molds ranged from 100 to 160CFU/g and for yeasts from 120 to 990CFU/g. Molds were found in honeys samples from the following states: 27.01% from Oaxaca, 25% from Michoacán, 21.3% from Jalisco, 20.8% from the State of Mexico, 15% from Morelos, 12.5% from Chihuahua, 8.3% from Mexico City and 6.3% from Yucatán. Yeasts were found in 29.2% of the honeys from Michoacán, 25% from the State of Jalisco, 23.3% from Oaxaca, 22.5 from the State of Mexico, 14.6% from Morelos, 11.7% from Mexico City, 11.3% from Chihuahua and 7.5% from Yucatán (Table 1).
With respect to coliforms, samples contained less than 3 NMP/g. For the other bacterial groups not considered in the standard for honey, we found that 2% contained LAB (39/1920), and 12% (247/1920) showed the presence of C. perfringens with more than 100CFU/g (Table 1). None of the samples from the different states exhibited more than 10CFU/g of S. aureus. Salmonella spp. was not detected in any of the samples.
Tables 1 and 2 show the presence of C. perfringens in the samples from each state: 21.3% for Michoacán, 19.6% for Oaxaca, 17.1% for Jalisco, 15.4% for the State of Mexico, 9.2% for Mexico City, 7.5% of Chihuahua and 6.7% for Yucatán.
Number of analyzed honey samples with pathogen or microbial group presence above 100 and 1000CFU/g
| Sampling place | MA >1000CFU/g | Molds >100CFU/g | Yeasts >100CFU/g | LAB >100CFU/g | C. perfringens >100CFU/g |
|---|---|---|---|---|---|
| Chihuahua | 87/240 | 30/240 | 27/240 | 0/240 | 18/240 |
| Ciudad de México | 62/240 | 20/240 | 28/240 | 5/240 | 22/240 |
| Estado de México | 120/240 | 50/240 | 54/240 | 8/240 | 37/240 |
| Jalisco | 128/240 | 51/240 | 60/240 | 12/240 | 41/240 |
| Michoacán | 117/240 | 60/240 | 70/240 | 6/240 | 51/240 |
| Morelos | 80/240 | 36/240 | 35/240 | 2/240 | 15/240 |
| Oaxaca | 136/240 | 65/240 | 56/240 | 6/240 | 47/240 |
| Yucatán | 47/240 | 15/240 | 18/240 | 0/240 | 16/240 |
| Totales | 777/1920 | 327/1920 | 348/1920 | 39/1920 | 247/1920 |
MA – mesophilic aerobic bacteria, LAB – lactic acid bacteria.
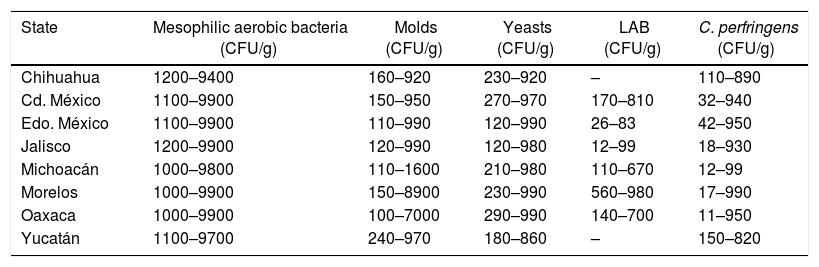
CFU intervals in samples above 100 and 1000CFU/g of honey per indicator group
| State | Mesophilic aerobic bacteria (CFU/g) | Molds (CFU/g) | Yeasts (CFU/g) | LAB (CFU/g) | C. perfringens (CFU/g) |
|---|---|---|---|---|---|
| Chihuahua | 1200–9400 | 160–920 | 230–920 | – | 110–890 |
| Cd. México | 1100–9900 | 150–950 | 270–970 | 170–810 | 32–940 |
| Edo. México | 1100–9900 | 110–990 | 120–990 | 26–83 | 42–950 |
| Jalisco | 1200–9900 | 120–990 | 120–980 | 12–99 | 18–930 |
| Michoacán | 1000–9800 | 110–1600 | 210–980 | 110–670 | 12–99 |
| Morelos | 1000–9900 | 150–8900 | 230–990 | 560–980 | 17–990 |
| Oaxaca | 1000–9900 | 100–7000 | 290–990 | 140–700 | 11–950 |
| Yucatán | 1100–9700 | 240–970 | 180–860 | – | 150–820 |
Forty point five (40.5%) percent from the 1920 analyzed honey samples exceeded the limits permitted by the regulation (NMX-036-NORMEX-200619), with bacterial counts higher than 1000CFU/g but lower than 10000 for the aerobic mesophilic count (Table 2). Similar studies in Argentina9, Brazil31, Colombia36, Costa Rica38, Croatia12, Portugal11 and Romania37 have determined that none of the samples analyzed exceeds 1000CFU/g of aerobic mesophilic bacteria and that they are below 100CFU/g of this indicator group as in Argentinian samples9. Moreover, 75% of Turkish samples showed counts ranging from 100 to 1000; however, the remaining 25% were between 1000 and 10000CFU/g. Nevertheless, none of these studies mention whether they comply with the normative standards of the country, although the study from Turkey mentions that the analyzed honeys have good microbiological quality7. Snowdon and Cliver30 mention that honeys can have an acceptable quality with a bacterial count up to 10000CFU/g of aerobic mesophilic bacteria if they comply with the criteria for yeasts and are free of fecal contamination.
For the counts of yeasts and molds we observed that 18.1% and 17% of the samples showed more than 100CFU/g respectively; in the studies conducted by Gomes et al.10; Iglesias et al.14; Gradvol et al.12; Vica et al.37; Zamora and Arias38, the counts for the group of molds were lower than 100CFU/g, however, in Turkey, 5% of the samples showed counts higher than 1000CFU/g and 10% higher than 100CFU/g for molds whereas only 20% of the samples were above 100CFU/g for molds35. In Brazil, 50% of the samples analyzed showed counts above 100CFU/g for yeasts and molds31.
Although honey has high osmolarity and low water activity, two factors that prevent the development of microorganisms, it may contain microorganisms that are found in several sources of contamination that are hard to control such as dust, air, soil and nectar. However, microbial contamination can also be caused by food handlers, equipment and crossed contamination. The latter can be controlled by standard sanitation and good manufacturing practices during honey harvesting and processing, otherwise, the honey would have as a consequence high levels of vegetative cells. Among the molds and yeasts involved in contamination, some genera such as Penicillium, Mucor, Saccharomyces, Schizosaccharomyces and Torula have been reported, which are responsible for the fermentation of honey when it shows a high degree of humidity (>21%)30.
All samples contained <3CFU/g of coliforms; for S. aureus no sample was above 100CFU/g, Salmonella spp. was not detected in the 25g of all the analyzed samples. Several authors mention that all the honey samples analyzed have shown absence of Salmonella spp. on the 25g analyzed of each sample and not more than 1 or 3CFU/g of coliforms, and none of these authors analyzed the count for S. aureus10,12,14,30,31,35,37,38. In 80 honey samples from Istanbul, positive samples for coliform counts, Escherichia coli and S. aureus were 16, 3.6 and 13.4% respectively, with a positive correlation of their presence among these microorganisms7.
Contamination by S. aureus is feasible due to an inadequate handling of honey. The authors mention that the presence of coliforms and Salmonella spp. can probably be attributed to fecal or environmental contamination7. Other authors mention that the presence of these bacteria can come from inappropriate premises, insects, material with a deficient process of de-contamination as well as extraction, packaging and storage9,15.
In situ we observed that in all sampled sites the handling of honey complied with the hygienic conditions for its processing and consumption no matter what production region of the Mexican Republic did the sample come from.
With regard to C. perfringens counts, we observed that 12% of the contaminated samples with this sporulating bacteria contained more than 100CFU/g of honey (Tables 1 and 2); the presence of this microorganism may be due to contamination by bees, nectar or external sources. There have been observations that the bee gut can contain up to 27% of gram-positive bacteria, some of which include several species of Clostridium, including C. perfringens.
It has been reported that spores of C. perfringens can survive and be maintained in a latent form for long periods of time at low temperatures when honey is inoculated with this microorganism3,16, therefore, the samples containing C. perfringens may have been contaminated in the apiary as a primary source of contamination through bees containing a sporulating microorganism in their guts. In honeys from Argentina, a low incidence of sulphite-reducing clostridia was reported15.
Furthermore, if honey is used for therapeutic purposes to heal wounds, spores of C. perfringens can germinate and proliferate causing problems of gangrene in deep wounds, although it has been observed that there are differences in bacterial survival. It has been reported that the use of honey prevents the wound from getting infected and can heal without risk of infection for up to 6 days although other authors report that bacteria can remain viable for 2, 3 and even 5 weeks23.
By the analysis and study of the therapeutic properties of honey, the medical significance of honey has been highlighted in the healing of wounds, ulcers, eye infections and burns13,18,33; currently, it has been used by some doctors for cancer care6. Its antioxidant and antimicrobial properties have also been considered1,10,29,34.
It is clear that the honey to be used for this latter purpose should be picked from areas where no sources of contamination exist8. Moreover, it is recommended that when honey is used, a previous microbial analysis should be carried out to avoid the contamination of wounds. It is also necessary to continue carrying out studies to establish factors affecting the standardization of honey, including source (monofloral or multifloral), type of honey (processed or raw), origin (natural or commercial honey), type and size of wounds to treat or the intended treatment2,13.
With regard to lactic acid bacteria, only 15.79% of the samples contained more than 100CFU/g (Tables 1 and 2). These kind of bacteria are considered to be safe (GRAS) and can play an important role in the preservation of the product since many of them have the ability to produce antimicrobial agents such as organic acid and bacteriocins that can inhibit or destroy pathogenic gram-negative and grampositive bacteria, although these characteristics may vary depending on the type of LAB. For the reasons already exposed, it may be an extra benefit to the properties of honey if it were to contain these acid lactic bacteria13.
The state that showed the highest percentage of samples with a high microbial count for aerobic mesophilic and molds was Oaxaca, and the one with less count was Yucatán. Samples from Michoacán were the ones with the highest count for C. perfringens. It is important to point out that Yucatán exports most of the honey in our country; according to these results, samples from this state are the ones with the best sanitary quality.
It is important to mention that over 40% of honey samples did not comply with the specification related to the presence of aerobic mesophilic bacteria that implies an emphasis on the handling and sources of contamination by local producers so that honeys can fit the standard. With regard to other microorganisms, we can conclude that the percentage of samples with more than 100CFU/g is less than 20% throughout the country, which can be the result of good sanitary handling of this food. It is important to avoid the sources of contamination and to follow good hygienic practices to maintain or improve the quality of the Mexican honey since a great percentage is exported. If honey is going to be used as a therapeutic agent, it should fulfill quality parameters and specific treatments to remove pathogens that can be threatening to the patients’ health should be implemented.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThere is no conflict of interest between the authors